Love in a time of Prozac; how did our emotions evolve?
Seeing red: for happy faces, or snakes in the grass?
Heated eyes give swordfish deep-sea ‘night vision’
Some 300m below the ocean surface it is always twilight, and cold… The water is barely above zero. Fast-moving squid hide here from predatory fish which stay near the surface; at this depth, their nerves would be so slowed by the cold that their eyes could no longer see for them to hunt effectively.
But there are exceptions; a stealthy predator dives into this semi darkness. Whilst the swordfish’s body temperature matches that of the water, its eyes and brain, crucially, stay toasty warm at around 23⁰C.
Why do swordfish have warm eyes?
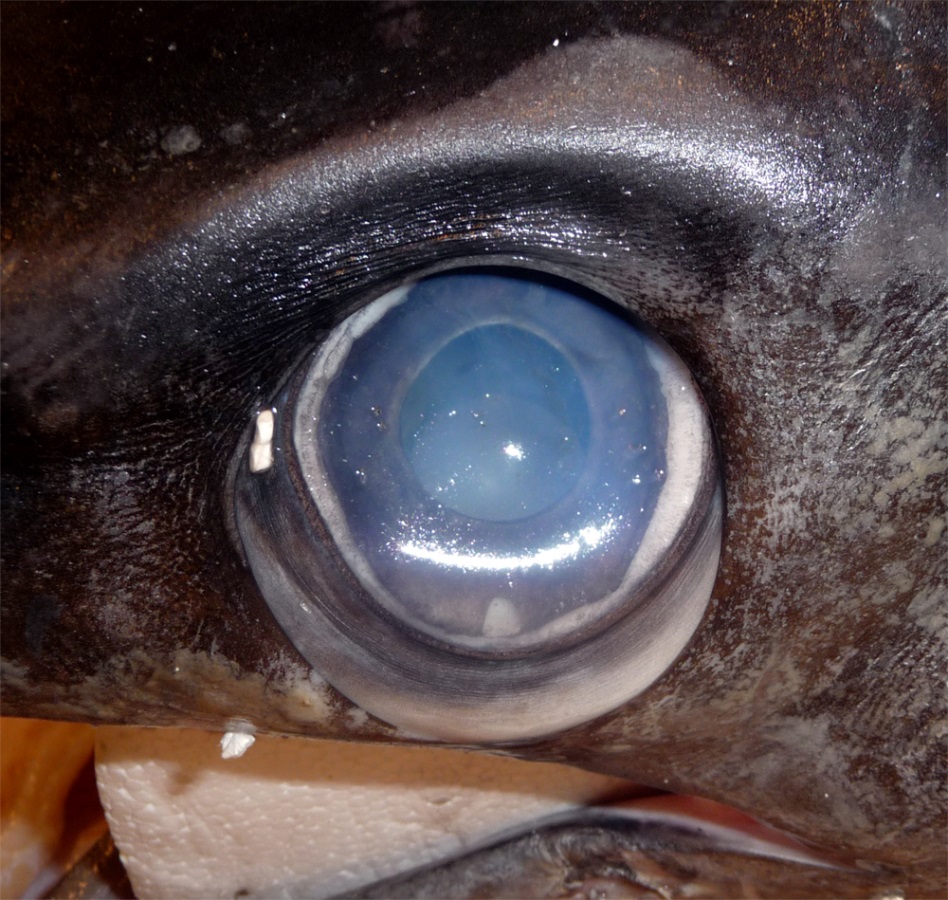
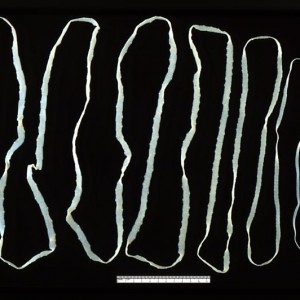
Close-up of a swordfish’s eye from a caught specimen. The eyes sit in a bony eye cup surrounded by a thick insulating layer of fatty tissue – part of which is visible here below the eyeball (Image: Wikimedia Commons... more)
A fish’s body temperature usually matches that of the water, meaning they are ‘cold blooded’ (poikilothermic). Swordfish nerves, like ours and those of other vertebrates, operate only within narrow temperature limits. The squid is also ‘cold-blooded’, but their elongated nerve cell axons however, are unusually wide, around 0.5mm diameter, and operate well in the cold, allowing them to maintain their fast movements and escape predators.
A few fish species, however, have evolved methods to generate heat in some of their tissues. Under the chilly, low light conditions of the deep sea, the warm eyes of the swordfish keep its optical nerve signals rapid. This allows it to register more visual signals per second than can other vertebrate predators. This fast image resolution ‘slows down’ apparent time and amplifies details, allowing these stealthy hunters to discern the brief flashes of silver that reveal the fleeting movements of small fish and squid.
This prompts some key evolutionary questions;
– How is the swordfish’s eye heat generated?
– How does the swordfish keep the heat localised to its eyes and brain?
– How does keeping body parts at different temperatures adapt swordfish for survival?
How is the swordfish’s eye heat generated?

Heat generation is not limited to animals. Some plants such as this Voodoo Lily (Amorphophallus titanium) have developed their own form of cellular heat generation, termed ‘non-shivering thermogenesis’. These unus... moreual plants heat parts of their floral organs to liberate scent messages into the air. This attracts insect pollinators, and may also protect its delicate reproductive tissues from the sometimes very cool night temperatures in its native tropical forest habitat (Image: Wikimedia Commons)
Swordfish eye muscles contain many brown-coloured cells that produce heat without shivering (non-shivering thermogenesis). They have a high metabolism (respiration rate) and contain many of the organelles known as mitochondria.
Mitochondria are formerly free-living bacteria found inside nearly all animal, plant and fungal cells. They ‘breathe’ for their cell, converting sugars and oxygen into carbon dioxide and water. This releases energy, which they use to pump hydrogen ions (H+, protons) from the internal matrix into their inter-membrane space. They use the chemical energy gradient this creates to produce adenosine triphosphate (ATP), life’s energy storage compound. These cellular energy factories are found in all animals and plants.
Humans and other mammals have brown adipose cells, also called ‘brown fat’. The mitochondria in these cells make very little ATP. Instead, ‘uncoupling proteins’ rearrange negatively charged fatty acids in the mitochondrial inner membranes to face into the inter-membrane space. These associate with the positively charged protons, then ‘flip-flop’, carrying them back into the matrix and dissipating the energy gradient as heat.
How does the swordfish keep the heat localised to its eyes and brain?

A pod of sperm whales (Physeter macrocephalus) diving off the coast of Mauritius. These animals are insulated by a thick layer of blubbery fat (Image: Wikimedia Commons)
When our bodies generate heat in a cold environment, this sets up an energy gradient; the bigger the differences between our internal and external temperature, the faster we cool. Warm bodies in a cold environment lose heat quickly, unless insulated. Birds use feathers, most mammals use fur and whales have blubber.
Fatty insulation over the swordfish’s skull retains heat, and helps keep its eyes and brain at a near constant temperature. These tissues are homeothermic (maintaining a stable temperature), whilst the rest of its body is poikilothermic (allowing temperatures to vary with the environment). Blood vessels supplying oxygen to the swordfish’s eye muscles are also arranged to retain heat. These vessels run in parallel, allowing outgoing veins to warm incoming arteries (this is known as a ‘counter-current’ heat exchange system).

Emperor penguins (Aptenodytes forsteri) at Atka Bay, Weddell Sea, Antarctica. The wide webbed feet of these birds have a large surface area. Reducing the skin temperature here reduces the steepness of the heat energy gr... moreadient at the place where their bodies contact the ice. This reduces the heat loss from these uninsulated body tissues (Image: Wikimedia Commons)
Insulation (fur, feathers or fat), combined with a blood supply arranged to allow counter-current heat exchange, are found in many cold-adapted animals. Lowering surface temperatures reduces the energy difference between a body and its surroundings, so minimising heat loss. Warm-bodied migrating species such as wolves and many birds from polar regions use a counter-current exchange to reduce the temperatures of their legs and feet. This means that their body parts in contact with snow or ice remain at just above zero.
How does keeping body parts at different temperatures adapt swordfish for survival?
Keeping your body at a different temperature from your environment requires a lot of energy. The swordfish’s ‘dual temperature’ body isolates the heat and keeps it in one well-insulated region; this is the most energy efficient way for these ‘wait and sprint’ hunters to survive in this environment. Tuna are another example of a fish with warm and cool tissues. Their red muscles along their spine are warm, and sustain constant ‘slow’ strokes of the tail during their long distance migrations.

An elephant dust-bathing in the ‘W du Niger’ trans-border national park, Niger Elephants cool down by ear flapping, and water and dust bathing. Their ears have a large surface area for their volume, and strong blood... more supply. Dilating the capillaries in the ears to increase blood flow to the skin allows these surfaces to lose heat to the air. At higher temperatures elephants lower their metabolic rate, reducing their resting body temperature (Image: Wikimedia Commons)
When we sweat, water evaporates and cools our skin surfaces. Dogs and many other mammals pant to evaporate water from their tongue and mouth cavity. Elephants lack both sweat glands and a panting reflex; these are possible remnants of their aquatic ancestry.
In very high temperatures they enter a whole-body heterothermic state. They slow their metabolism, lowering their morning body temperature. They then absorb daytime heat, raising their temperature above 36.7⁰C, and radiate this ‘stored’ heat at night.
Varying the temperature at times like elephants, or in certain tissues like swordfish, is known as heterothermy.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Carey, F.G. (1982) A brain heater in the swordfish. Science 216, 1327-1329.
Fritsches, K.A. et al. (2005) Warm eyes provide superior vision in swordfishes. Current Biology 15, 55-58.
Guderley, H. et al. (2005) Why are some mitochondria more powerful than others; insights from comparisons of muscle mitochondria from three terrestrial vertebrates. Comparative Biochemistry and Physiology, B 142, 172-180.
Hulbert, A.J. et al. (2006) How might you compare mitochondria from different tissues and different species? Journal of Comparative Physiology, B 176, 93-105.
Kowaltowski, A.J. (2000) Alternative mitochondrial functions in cell physiopathology; beyond ATP production. Brazilian Journal of Medical and Biological Research 33, 241-250.
Nespolo, R.F. et al. (2011) Using new tools to solve an old problem; the evolution of endothermy in vertebrates. Trends in Ecology and Evolution 26, 414-423.
Warrand, E.J. and Locket, N.A. (2004) Vision in the deep sea. Biological Reviews 79, 671-712.
Weissenbrock, N.M. et al. (2012) Taking the heat; thermoregulation in Asian elephants under different climatic conditions. Journal of Comparative Physiology, B 182, 311-319.
Making waves; how moving our arms as we talk signals our ‘inner fish’
The old Jewish Cemetery; Venice, 1790.
Goethe loosens the earth from the skull, and holds it up to the sun.
Turning the fractured bone back and forth, he gasps. A series of marks appear inside the cavity, reminding him of the vertebrae. He has looked at this pattern many times, but without seeing what lights up before him today.
Here is the shadow of a blueprint; the ‘primal repeating units’ of animal bodies, from which their many variations form.
Puzzled, Götze watches his master’s eyes shine with delight.
Watch this baby babbling; her limbs move, often in time with her sounds. the coupling of gestures and vocal calls are widespread amongst social vertebrates. And the story began with fish.

Watch this baby babbling.The rhythmical arm and leg movements of human infants as they vocalise reveals some ancient neural wiring, inherited from our common vertebrate ancestors, and now shared with other modern verteb... morerates from elephants through reptiles, amphibians and birds to fish (Image: Wikimedia Commons)
The rhythm of our breath keeps us alive. Conscious muscle movements are made through spinal nerve reflexes, but like our heart beats, the repeating sequences of muscle actions which fill and empty our lungs are outside our conscious awareness.
The movements behind repetitive activities like breathing are driven by rhythmic nerve impulses from ‘neural oscillators’. These pattern-generating circuits, located in the central nervous system, are known as ‘Central Pattern Generators’.
To breathe, to speak and to swallow we use the same internal tube; that is our throat (the pharynx). These activities are necessarily exclusive; consider what happens when a crumb ‘goes down the wrong way’. Speech therefore needs to be coordinated with our breathing.

The male club-winged manakin (Machaeropterus deliciosus) from the cloud forests of Ecuador makes sounds by rapid wing vibrations. This rhythmic movement is driven by the vertebrate vocal central pattern generators. The ... moreline drawing (shown right), from Charles Darwin’s book The descent of man, shows how the male birds’ secondary flight feathers (top ) are modified for sound (the equivalent feathers from the female bird are shown in the bottom row). Watch. (Images: Wikimedia Commons)
We make vocal sounds by passing air through the larynx as we breathe out, at the same time as vibrating our vocal folds (vocal cords). These actions involve coordinating a sequence of repetitive movements inside the throat with the repeating muscle actions that drive our breath.
Communicating with sound evolved long before animals emerged from the sea onto land and began to breathe air. Many fish use pectoral fin movements as communication gestures; some also generate sounds by fin waving.
In species of vocal fish, the calls are coordinated with these pectoral fin signals. Significantly, the muscles operating these social communication cues are controlled using the same neural oscillator ‘module’.
‘Central Pattern Generators’ are neural oscillators that generate a rhythmic output, used to control repeating muscle movements. These ‘neural metronomes’ were first discovered in insects, and produce their steady pulse without any sensory stimulus. In contrast, our other nerves operate on a ‘stimulus-response’ basis.

The predictable and repetitive movements we use for breathing, chewing and walking can speed up and slow down, but the sequence in which these muscles work (the oscillatory cycle) does not change. Oscillators are known ... morein mechanical, chemical and biological systems. This simple (undampened) oscillating spring can alter its speed, but the nature of the movement remains the same (Image: Wikimedia Commons)
Central Pattern Generators reveal what can be called our ‘deep homology’. First discovered in insects, all vertebrates, including ourselves, have these ancient neural circuits. They links vocal calls with gestures, and coordinate our ‘fins’ with our speech.
How do Central Pattern Generators work?
We consciously control our limbs through spinal nerve reflex arcs. In contrast, rhythmic movements controlling oscillating cycles are driven by Central Pattern Generators (CPGs). These autonomous modules in the central nervous system produce a rhythmic output (like a neural ‘black box’). CPG modules are comprised of a dense interconnected local network of neurons; a neural ‘node’.
These nodes are organised into three levels, each with a different function, and in each case, the parts of the circuit ‘higher’ in this organisation regulate the outputs of those below.
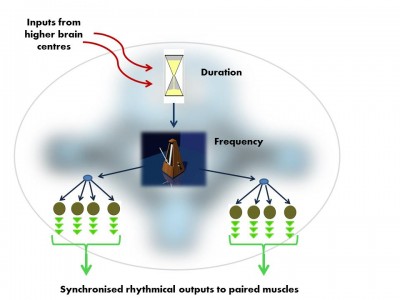
The vocal Central Pattern Generator used to produce basic signals for social communication, is organised in much the same way in fish, frogs, birds and mammals. Like all Pattern Generator modules it has a hierarchical o... morerganisation.i. pre-pacemaker cells set the duration of the output,ii. pacemaker neurons set the frequency of the regular nerve impulseiii. Motor neurons transmit the pacemaker’s rhythmic output to the muscles (Image: Wikimedia Commons)
In the developing embryo there are functional units, ‘segments’ which give rise to our vertebrae and their associated nerves and muscles. The nerves from each of these ‘segments’ form our local sensory spinal reflexes and also the CPG modules. As needed, higher brain centres trigger these ‘neural motors’ to produce their rhythmical nerve impulses and drive all of our rhythmical movements from walking to chewing.
CPGs controlling rhythmic movements of the tongue, throat and breathing (including the vocal neural oscillator module) are in the lower brainstem and neck. The CPGs that drive the rhythm of our walking are low down in the spinal cord, in the thoracic and lumbar regions.
Why don’t we sound like fish?

Vocal fish such as this Oyster toadfish (Opsanus tau) produce calls in one of two ‘output modes’. This is controlled by testosterone, which reduces the threshold of nerve stimulus needed to initiate calls. In ‘nor... moremal’ mode, these fish are able to sustain only slow rhythmic grunts. ‘Mating mode’ speeds up these sounds into a buzzing drone. Mating calls are made only at night during the spawning season, when testosterone levels are high.In this video clip the closely related plainfin midshipman fish (Porichthys notatus) demonstrates both call types (Image: Wikimedia Commons)
Toadfish vocalise with either a sequence of repetitive grunts during aggressive encounters or the prolonged drone of their mating call. In both cases, each nerve impulse from the vocal pattern generator produces a single synchronised contraction in their sonic muscles; this muscle pair flexes the rigid walls of the swimbladder, producing a ‘grunt’. This sound receives no further processing. As a result, its tone is rather mechanical.
Our voice, like that of frogs, birds and mammals, also begins with this simple rhythmic sound pulse. This initial sound is then processed into croaks, calls songs and speech. Our neck allows us to create resonant areas in the throat which amplify certain frequencies. Pitch is affected by vocal fold (vocal cord) tension, and manipulation of our tongue and lips produces precisely articulated words.
Why is ‘talking with our hands’ still a part of our language?

This Siamese fighting fish (Betta splendens) uses rapid pectoral fin movements as a posturing signal during competitive displays with other males. Watch displaying fish in adjacent tanks using pectoral fins signals (Image: Wikimedia... more Commons)
Many vocal fish make synchronised gestures with their front (pectoral) fins during mating calls. These motor nerve connections from our ancient common ancestor are retained in other vertebrates.
People blind from birth move their hands when they talk. Our vocal Pattern Generator circuits connects with both our larynx and pectoral muscles, coordinating our speech with our ‘body language’. We subconsciously move our hands as we communicate thanks to these rhythmic central circuits. As in all other vertebrates, we have inherited these from (as Palaeontologist Neil Shubin puts it) our ancestral ‘inner fish’.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Aboitiz, F. (2012) Gestures, vocalizations, and memory in language origins. Frontiers in Evolutionary Neuroscience 4, e2.
Bass, A.H. and Chagnaud, B.P. (2012) Shared developmental and evolutionary origins for neural basis of vocal-acoustic and pectoral-gestural signalling. Proceedings of the National Academy of Sciences, USA 109 (Suppl.1), 10677-10684.
Bass, A.H. et al. (2008) Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science 321, 417-421.
Bostwick, K.S. (2000) Display behaviors, mechanical sounds, and evolutionary relationships of the Club-winged Manakin (Machaeropterus deliciosus). Auk 117, 465-478.
Bostwick, K.S. et al. (2010) Resonating feathers produce courtship song. Proceedings of the Royal Society, B 277, 835-841.
Chagnaud, B.P. et al. (2012) Innovations in motoneuron synchrony drive rapid temporal modulations in vertebrate acoustic signalling. Journal of Neurophysiology 107, 3528-3542.
Dick, A.S. et al. (2012) Gesture in the developing brain. Developmental Science 15, 165–180.
Ghazanfar, A.A. (2013) Multisensory vocal communication in primates and the evolution of rhythmic speech. Behavioral Ecology and Sociobiology 67, 1441-1448.
Goethe, J.W. (1820). Zur Naturwissenschaften überhaupt, besonders zur Morphologie; cited (p. 7) in G.R. de Beer The Development of the Vertebrate Skull. Clarendon (1937).
Guthrie, S. (1996) Patterning the hindbrain. Current Opinion in Neurobiology 6,41-48.
Hanneman, E. et al. (1988) Segmental pattern of development of the hindbrain and spinal cord of the zebrafish embryo. Development 103, 49-58.
Iverson, J.M. and Thelen, E. (1999) Hand, mouth and brain: The dynamic emergence of speech and gesture. Journal of Consciousness Studies 6, 19-40.
Kelley, D.B. and Bass, A.H. (2010) Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Current Opinion in Neurobiology 20,748-53.
Marder, E. and Bucher, D. (2001) Central pattern generators and the control of rhythmic movements. Current Biology 11, R986-R996.
Shubin, N (2008) Your inner fish; a journey into the 3.5 Billion year history of the human body. Penguin Books Ltd.
Shubin, N. et al. (2009) Deep homology and the origins of evolutionary novelty. Nature 457, 818-823.
A sense of protection: is the immune system an extension of our minds?
Entering a body is much like entering a country.
At the border, agents verify the identity of those seeking to pass, and establish their status as ‘native’, a ‘naturalised resident’, or ‘alien’. Clarity about the identity of these travellers allows them to be appraised as ‘friend’ or ‘foe’.
The body of a nation operates best with safety measures in place. Its borders are a first line of surveillance that ascertains when to mobilise defences.
Border checks in some countries are more efficient; they deploy new technologies to implement higher orders of monitoring and identification.

Scanning electron microscope image of a neutrophil, a cell from the innate immune system (yellow), engulfs anthrax bacteria (orange); scale bar = 5 micrometres.Body cells that experience stress, wounding, or sense the p... moreresence of bacteria, produce cytokines and other signals that trigger the innate immune response, and attract immune cells into the tissues.Some of these cells (‘phagocytes’) like this neutrophil, engulf and digest the invaders, whilst others (‘granulocytes’) secrete granules of cytokine, histamine and various types of oxygen free radicals, all of which enhance the inflammation response. Proteins in the blood, the ‘compliment system’, also condense onto and coat unwanted bacterial invaders and other particles. This targets them for destruction by phagocytes, or removal from the blood stream in the spleen (Image: Wikimedia Commons)
Typically we think of the immune system as a defence mechanism; the forces we mobilise to fight disease. Animals, plants, fungi and microbes all have a form of this type of defence. Even bacteria have a version of immunity, using enzymes that can react to and then digest viral proteins.
Immune responses in animal systems are thought of as having two levels of action.
Innate immunity
First, ‘innate immunity’ provides a spectrum of responses which use secreted proteins and dedicated cell types to target common components of a broad range of diseases.
Amongst animals, invertebrate bodies are relatively simple. In contrast, vertebrates have increased genetic and physical complexity, more sophisticated sensory perceptions and cognitive processing and, in some clades, enhanced social organisation and communication. This increased complexity is reflected in the vertebrate immune system.
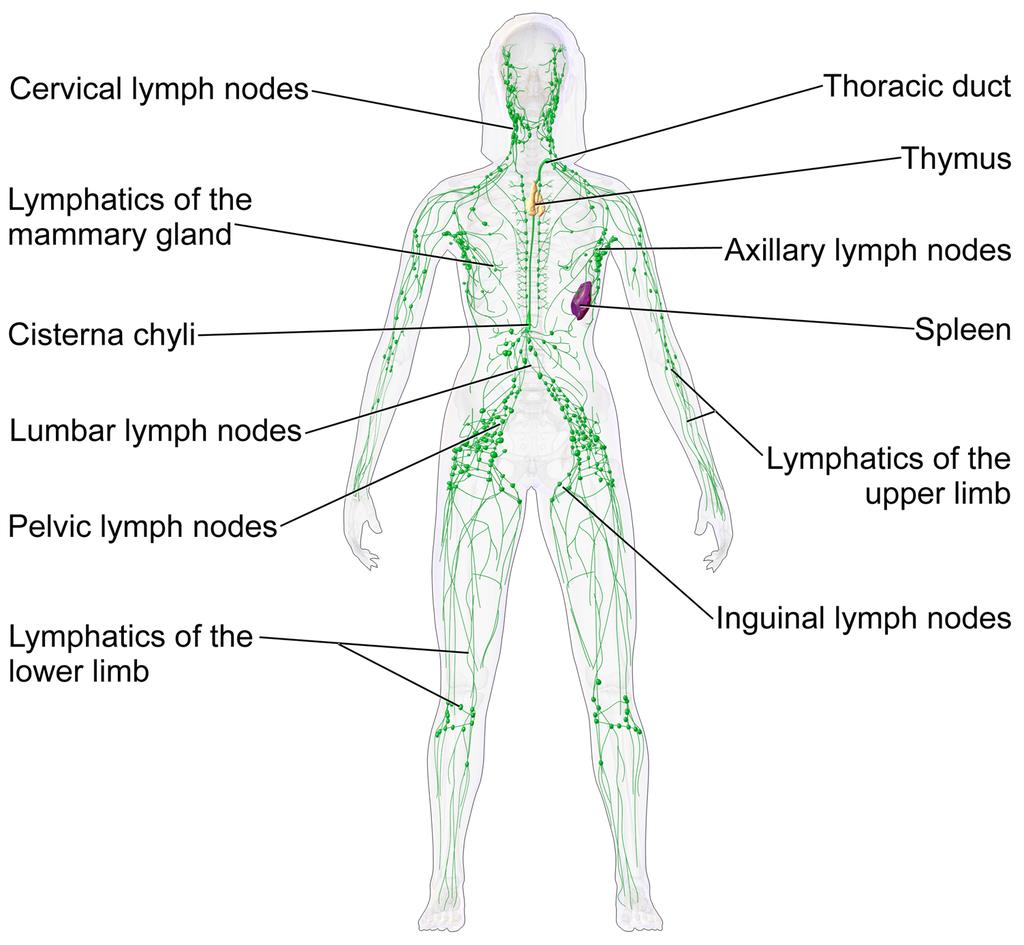
The lymphatic system of a human female. This network of vessels allows immune system cells rapid access to all areas of the body. Lymph vessels interconnect with the blood circulation at the heart and lymph nodes.Two or... moregans are particularly important in this network.• The thymus gland polices antibody-producing adaptive immune system cells, filtering out those which are too close to recognising native body proteins.• The spleen synthesises antibodies in its white pulp, and releases these proteins into the blood stream, where they bind to foreign proteins, such as those present on the surface of bacteria. This targets cells and other debris in the blood. The spleen removes antibody-coated bacteria and old red blood cells from the circulation. It also acts as a reservoir for monocytes, immune cells which later develop into phagocytic (non-specific engulfing) cells (Image: Wikimedia Commons)
Adaptive Immunity
The second form of immune response, known as ‘adaptive immunity’, refers to the ability to identify and target specific invaders. In vertebrates, this immune function first appeared in jawed fish.
In broad terms, it involves three phenomena.
– A new immunological organ (the thymus).
– A repertoire of specialised lymphocyte cells including T-cells (matured in and released from the thymus gland) and B-cells (released from the bone marrow), some of which produce antibodies.
– The production of a ‘self-identity’ signature, the ‘Major Histocompatibility Complex’ (MHC) proteins, in all body cells.
The community of bacteria associated with our gut, lungs and other surfaces comprise the first line of defence. This community of microbes (our ‘microbiome’) out-competes many harmful agents (pathogens).

Like the gut, our lung linings have a microbiome. Both ’friendly’ microbes and infective agents (here Streptococcus pneumoniae and Pseudomonas aeruginosa) produce proteins and other chemicals that act as signals. C... moreells lining our lungs act as signal receivers and recognise these chemicals, producing cytokines and other cues which relay messages to the immune system.(Image: Wikimedia Commons)
If our body cells become infected or damaged, they release stress signals such as oxygen and nitrogen free radicals and various peptides (short strings of amino acids) such as cytokines and defensins. These cues act as messages to the immune system, potentially triggering inflammation and other chemical defences which attract immune cells into the tissue by chemotaxis.
The microbial community also produces these immune-stimulating signals, along with brain-active chemicals (neurotransmitters). In addition, these signals are also generated, recognised by, and responded to by immune system cells.

Diagram of an antibody. These immune system proteins are a complex of four separate protein chains. In this diagram, blue regions are consistent whilst the yellow regions are variable. They are secreted into the blood s... moretream or gut. Adaptive immune cells use them as ‘antennae’. When this signal receiver encounters an antigen that is a good fit, this triggers an adaptive response. All proteins interact by shape. Antibodies have a recognition region which is highly variable. The strongest immune reactions provide a ‘best fit’ to this three-dimensional molecular jigsaw. (Image: Wikimedia Commons)
Our neurons and immune cells both respond to hormones within our bodies, since chemically these are close to neurotransmitters. This means that our brain, hormonal system, sensory nerves and extended microbial community all share a common chemical language with the innate immune system, present in all animals.
The greater the biochemical diversity of cell populations in a body, the more opportunities there are for bacteria or viruses to find novel ways of attacking these cells. For this reason, adaptive immune system cells themselves are vulnerable to certain types of disease. For instance AIDS (Acquired Immuno-Deficiency Syndrome), caused by the Human Immuno-deficiency Virus (HIV), specifically targets and infects the adaptive immune system’s T-cells, using the very mechanism that allows these cells to detect infection – their surface antibodies.
Potentially then, adaptive immunity provides a much more sophisticated and versatile immune mechanism. For vertebrates with their adaptive system to be ultimately more vulnerable to certain infections than the innate-only immunity of invertebrates seems bizarre and inefficient. Yet adaptive immune systems have evolved independently twice in vertebrates. This suggests that the adaptive mechanism must convey some survival advantages, but disease resistance may not be the primary role which has driven its evolution.
How does the system work, and what is the real purpose of adaptive immunity?
Adaptive mechanisms give our immune system a ‘memory’
In vertebrates, ‘adaptive immunity’ adds an additional higher-order function to the innate immune system by providing a means to both recognise and ‘remember’ specific diseases. To do this, they use a protein recognition system; these are the familiar antibodies.
These proteins have a shape-specific region which fits the shape of part of a foreign protein (an antigen) like a key in a lock. An antigen is a molecule that is capable of triggering an immune response; this can be from a foreign source (e.g. a virus) or produced by an unhealthy body cell, e.g. a cancer cell.

The adaptive immune system “adapts” to infections that get past our innate defences. Phagocytic macrophage (innate system) cells behave like amoebae, and can engulf and digest foreign bacteria. These ce... morells become ‘antigen presenting cells’ (APC), and displaying short pieces of bacterial protein (the antigen) on their cell surfaces, along with a self-signal, the ‘Major Histocompatibility Complex’ class II protein (MHC2). The MHC tells the immune system that this cell is a messenger, not an invader. These two proteins together activate the adaptive immune system.There are two types of adaptive response. First, T lymphocytes (T cells) display an antibody on their surface that recognises the foreign fragment on an APC. They then produce other surface proteins (here CD4+) that signal a change of identity, turning them into ‘‘helper’ cells. Helpers can provoke macrophages or B lymphocytes (B cells) to act. An activated B cell releases antibodies into the blood stream that ‘seek out’ and identify the invader. T cells can also become ‘killers’ that recognise and destroy infected body cells. (Image: Wikimedia Commons)
Adaptive immune cells called T-cells produce antibodies and ‘display’ them from their cell surfaces, using them as ‘antennae’ to detect invaders. Phagocytic (engulfing) innate immune cells can act as antigen presenting cells, displaying foreign protein fragments (antigens) on their surfaces. T-cells whose antibody ‘fits to’ this antigen can then be triggered to respond.
Triggered T-cells divide multiple times, so producing identical clones of themselves. This increases the magnitude of the body’s immune response to the infection. Activated T-cells relay the signal in turn to adaptive B-cells, triggering them to secrete large amounts of antibody proteins specific to this foreign antigen into the blood. These bind to the foreign antigens, coating the virus particles or foreign bacteria in antibodies. This targets the innate system’s macrophages to ‘engulf and destroy’.
Immune system cells interact with each other in a similar way to nerve cells
The association between the antigen presenting cell and the activating T-cell is very close, and the trigger mechanism transmits a one-way signal between the cells. This is very like how a nerve synapse transmits a signal to the next nerve. It is therefore entirely reasonable to call it an ‘immunological synapse’.
This between-cell interaction, which here involves a specific recognition between the immune cells, arises from an ancient, general mechanism of between-cell communication that allows signal-transmitting contacts to form. Activated T-cells initially show pulses of electrical activity, produced by a release of calcium ions (which carry an electrostatic charge) into the cell matrix (cytoplasm).
Synapses are stable contacts that form between two distinct cells that allow for information transfer through directed secretion. Synapses between neurons in the brain form relatively stable contacts, although these remain ‘plastic’, able to be recruited into new neural networks and adopted for new purposes.
In the immune system, ‘synapse’ formation between for example an antigen presenting cell and a T-cell, provokes the T-cell to initiate a signal relay transmitted to other cells which produces a range of specific ‘actions’. These responses, resulting in defences that target the specific foreign antigen, are produced by cells further downstream of this initial ‘synapse’.

When a patient is first vaccinated, the adaptive immune system raises a response against the foreign antigens. These cells persist at a high level for several weeks, then the antibody level and effector T-cell activity ... moregradually declines. When a patient is re-vaccinated, a stronger response results. Selection favours T-cells with small differences in antibody binding that give a stronger reaction. These highly specific clones remain in the serum and lymphatic tissues and provide protective immunity against reinfection by the same agent. This gives a longer term immunological memory; later reinfection leads to a rapid increase in antibody production and effector T cell activity, which often prevents the disease from being apparent (Image: Wikimedia Commons)
Vaccinations activate our body ‘memory’
By cloning and maintaining a population of cells with disease-specific antibodies, our immune system ‘remembers’ how it responded to the disease. A second exposure produces a refinement of the accuracy of the recognition mechanism, and raises a population of ‘memory T-cells’ that persist in the body for much longer. This is the basis of vaccination. Exposing our immune systems to proteins from the infective agent, via an injection, allows us to develop resistance in a controlled, safe way. Our bodies raise a population of immune cells that ‘remember’ how to recognise these foreign proteins. If we later meet the disease, these ‘memory cells’ activate, enabling us to more quickly overcome the infection and contract only a mild version of the disease, or perhaps experience no symptoms at all.
These mechanisms suggest that adaptive immunity should provide enhanced resistance to diseases beyond what the innate response alone can deliver. In practice, however, invertebrates seem to be vulnerable to fewer diseases than we are. This may be a result of their simpler and less diverse profile of cell and tissue types.

Diagram of an HIV particle. Using cell surface antibodies as an infective agent identification mechanism means that immune cells can in turn become ‘visible’ to these pathogens. The Human Immunodeficiency Virus (HIV... more) which causes Acquired Immuno-defficiency Syndrome (AIDS) specifically identifies T-cells. This virus is a master of disguise; it uses a similar gene shuffling mechanism as are used to produce variable proteins on the surface of its viral capsule. This changeability makes the virus seem to always be something new – this is the reason that the immune system has difficulty recognising and neutralising it, and also is why the virus has been so difficult to target using a vaccine. The interaction between an infection and the host immune system is usually like a predator prey relationship. The immune system is the ‘predator’, chasing down to consume the infection (the ‘prey). In the case of HIV, these roles are unclear. Is the virus pursing the immune cell, or is the immune cell pursuing the virus? (Image: Wikimedia Commons)
Why would vertebrates evolve this expanded complexity in their immune system?
Every living thing, including plants and bacteria, possess an immune system of some kind. What we recognise as innate immunity is found in plants and also throughout the animal kingdom. Adaptive immunity raises the degree of complexity. Significantly, it has evolved twice independently within the vertebrates; in jawed vertebrates (sharks to humans) and also in the more ‘primitive’ jawless clades. Certain invertebrate groups (e.g. snails and insects), and indeed also bacteria, have a quasi-adaptive system, using specific, cross-reactive molecules, although these systems are not as sophisticated as that found in the vertebrates.

A river lamprey (Lampetra fluviatilis) from the German North Sea. The immune systems of this and other jawless fish such as the hagfish have been found to contain adaptive cells which act in a similar way to T- and B- l... moreymphocytes of the jawed vertebrates. Here the membrane proteins acting as signal receivers are different, based on receptors containing leucine-rich motifs. As in their jawed cousins, the immune responses of lampreys produce a clonal increase of specific disease-detecting cells (Image: Wikimedia Commons)
Studies in the jawless fish, lamprey and hagfish, show that a very different form of adaptive system has arisen than is found in jawed vertebrates, co-opted from a different set of genes and proteins. In contrast, their immune molecules include proteins with repeated sections in the chain which have multiple residues of the amino acid leucine (termed ‘Leucine Rich Repeats’, or LRRs). A form of LRR-based adaptive immunity is also found in plants.
Jawed vertebrate adaptive immunity however is rather more developed than that of the jawless fish. Jens Rolff makes two intriguing suggestions as to what may have driven its evolution.
i. Vertebrate bodies are larger and more complex than invertebrates, providing a new set of potential habitats for parasites. Rolf suggests that an evolutionary ‘arms race’ may have occurred between vertebrates and a group of parasitic Platyhelminthes, which include tapeworms and liver flukes. These parasites began to diversify after the evolutionary divergence between jawed and jawless fish.
The surface of these parasites is highly resistant to our immune defences, suggesting that they co-evolved in tandem with an immune-competent host. The increasingly more aggressive and targeted immune response required from the host to expel these parasites may have selected for the direct recognition mechanism and targeted responses of adaptive immunity. This theory also implies that the adaptive components of the immune system originally evolved in association with the gut.
ii. Vertebrates have higher metabolic rates than invertebrates. This allows for greater rates of activity and access to a wider range of ecological habitats. Higher energy bodies require more sophisticated brains and nervous systems, and have higher maintenance demands.

Scheme showing a DNA ligase-I enzyme (in color) repairing a break in DNA. DNA damage, caused by environmental factors and normal metabolic processes, occurs at a rate of 1,000 to 1,000,000 molecular lesions per cell per... more day. Without maintenance to mend such breaks, cells can malfunction, die, or become cancerous. Ligases catalyse the crucial step of joining the breaks in the spiralling DNA strand. To do so they require energy, in the form of either Adenosine triphosphate (ATP) or Nicotinamide adenine dinucleotide (NAD+) as a cofactor (Image: Wikimedia Commons)
Rolff suggests that the adaptive immune system is part of the normal homeostasis of a higher energy metabolism. Such regimes also produce higher concentrations of oxygen free radicals and other stress-associated compounds which can damage DNA and cause cancers. High energy vertebrate bodies therefore require a means of self-monitoring. The adaptive immune system may provide just such a set of ‘internal eyes and ears’.
How does the adaptive immune system function in vertebrates?
Our immune system discerns ‘friend’ from ‘foe’, ‘self’ from ‘non-self’ and monitors the status and identity of our own body cells. The adaptive system provides specific ways to identify and monitor foreign and native body cells.
An immunological synapse relay is triggered when for example a T-cell antibody recognises a foreign protein on an ‘antigen presenting cell’. This and other types of immunological synapse require a class of proteins related to antibodies; the ‘Major Histocompatibility Complex’ (MHC) proteins.
Vertebrates produce and display MHC proteins on their cell surfaces. During an infection, if a virus or microbial pathogen enters a body cell, it becomes invisible to the immune system. Infection, however, alters the health of the cell, reducing its surface MHC production. Reduced or absent MHC triggers ‘Natural Killer’ (NK) cells to form an immunological synapse with them, this time triggering the destruction of the infected body cell, and helping to reduce the spread of the disease.

As a cell is first infected by a microbial pathogen, (as in this diagram), it quickly mobilises its protein-dismantling enzymes (the ‘proteasome’) to digest the foreign proteins into short fragments (peptides). Spec... moreialised pores (‘Transport of Alien Protein’ or TAP channels) capture the fragments and shunt them into the endoplasmic reticulum, where the cell assembles its own proteins. Here the fragments are bound to ‘Major Histocompatibility Complex’ class 1 (MHC I) proteins. These are transported to the cell surface where they protrude like a beacon, presenting the foreign protein to the innate immune system’s ‘Natural Killer’ cells (Image: Wikimedia Commons)
MHC proteins also act as antigen presenting molecules. At an early stage of infection, the cell’s own defences may digest some of the invading pathogen’s proteins. These foreign antigens can be bound by MHC molecules, and presented on the cell surface. Again, this triggers NK cells to target this body cell for destruction.
Antigen-presenting phagocytic immune cells also do this using a different class of MHC protein, which avoids them activating the NK cells.
Recent research has also shown an unexpected role for MHC proteins in neuronal plasticity. Brain neurons require MHC class I molecules to establish new neural networks. Along with a range of other immune system receptors, these molecules are critical for the construction of new connections and the remodelling of synapses. This suggests that the transmission of a signal across the neural synapse and the immunological synapse are likely using the same mechanism.
What does this suggest as the primary role of adaptive immunity?

The ‘molecules of emotion’ with their effects. The mood effects that result from their deficiency in italics. These neurotransmitters are the same chemical language as is used by cells of the immune system (Image: W... moreikimedia Commons)
Candace Pert’s work demonstrated that immune cells both respond to and produce the same neurotransmitter signals as brain neurons. She initially found opiate receptors from the brain in immune system cells. Opiates are drugs that trigger the body’s responses to endorphins, the body’s natural ‘feel good’ chemicals. Pert showed that receptors for neurotransmitters which affect our mood; the ‘molecules of emotion’ including b -endorphins and serotonin, are present in immune system cells. Immune cells also manufacture these same chemicals.
Immune cells are everywhere in the body. Brain-based phenomena such as depression impact the function of the immune system such that our level of disease resistance rises and falls with our moods. In at least chemical terms, these cells are operating in conjunction with our neural network. Pert’s results reveal that through the immune system, our minds occupy the full sensory space of our bodies, and are not limited to our brains. By the same route, immune cells convey the chemical implications of our emotional state into every cell in our body. This means that the way we think has a direct impact on our health.
Adaptive immune cells recognise stress signals, neurotransmitters, and foreign proteins. Foreign antigen recognition activates the immunological synapse, and triggers the adaptive immune response. Recent research has shown that the MHC proteins, essential for this response, are also involved in the functioning of nerve synapses in the brain. This implies that the two systems are really both part of the same ‘neural super-organ’.
This ‘organ’ has flexibility as to where it focusses its attention. Release of cytokines and other stress signals draw immune cells into tissues where their surveillance is most required. This roving internal sense organ has been referred to by Enzo Ottavani and Claudio Franceschi as an ‘immune-mobile brain’ whose ‘eyes and ears’ are our adaptive immune cells.

Poster, emphasising the importance of surveillance, issued by the office for Emergency Management in the United Kingdom during the Second World War (Image: Wikimedia Commons)
This surveillance system cross-reacts with and can mutually influence the body’s hormonal system and gut microbiome. Edwin Blalock has suggests a primary role for the immune system as a sense organ; truly our ‘sixth sense’, which is able to detect stimuli not recognised by the central or peripheral nervous system. This information, the chemical language of the body’s bacterial community, is translated into a cognitively accessible form by the antennae of the adaptive immune system.
This provides a picture of the brain and immune system as forming an integrated ‘body-mind’. This system’s sensory perceptions, thoughts, emotions and behavioural responses impact the physical body at the biochemical level. This ‘embodiment’ idea has also become apparent through recent insights into the actions of mirror neurons and the neural basis of human language.
Having a conversation in the same language synchronises our minds. The brain and immune system translate this into hormonal and other chemical cues of emotion.
This suggests that our immune system is one part – a subconscious part – of our overall consciousness. This consciousness is integrated into our ‘body-mind’. Through sharing our thoughts with others, our awareness operates beyond the body at a higher level, and as we do this, we connect at the level of our chemistry.
Conclusions
- Innate immunity is general, and present in all cells in some form, from bacteria to humans.
- Adaptive immunity, where the organism raises a specifically cross-reacting chemical response to an infection, has evolved multiple times in multicellular life forms, including twice in vertebrates. Characteristics of adaptive immune systems in vertebrates are the appearance of cross-reacting proteins (antibodies) produced by specialised immune cells, a self-identifier (the major histocompatibility complex) in all body cells, and a thymus gland, which is a new organ within the lymphatic system.
- The driver of immune system evolution in vertebrates is not primarily defence. Factors that have driven the evolution of immune complexity in the vertebrate system (relative to invertebrates) may include (i) evolutionary ‘arms races’ with gut parasites, and (ii) an increased need to monitor the metabolic by-products of the higher energy vertebrate body.
- Immune complexity seems to be linked to the complexity of the overall organism, and fulfils a self-surveillance role. The increased complexity of vertebrate systems has in fact made them vulnerable to a wider range of infections than invertebrates.
- Vertebrate immune cells, neurons, and body cells all share a common chemical language; a language also used by the associated microbes that live on and around the vertebrate body (the microbiota).
- Immune cells form synapses, using a similar between-cell communication apparatus that neurons use to form their synapses. However the immune synapses are more transient, and this component of the body’s relay network is able to migrate to tissues where it is needed, augmenting the body’s surveillance in this area.
- Brain-body communication is two-way. The immune system integrates our thoughts and emotional state with our body, as well as informing the mind of the status of the body’s cells and tissues.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Abedon, S.T. (2012) Bacterial ‘immunity’ against bacteriophages. Bacteriophage 2, 50–54.
Bellinger, D.L. and Lorton, D. (2014) Autonomic regulation of cellular immune function. Autonomic Neuroscience 182, 15-41.
Bhalla, A.K. (1989) Hormones and the immune response. Annals of the Rheumatic Diseases 48, 1-6.
Bhat, R. and Steinman, L. (2009) Innate and adaptive autoimmunity directed to the central nervous system. Neuron 64, 123-132.
Blalock, J.E. (2005) The immune system as the Sixth Sense. Journal of Internal Medicine 257, 126-138.
Blalock, J.E. (2002) Harnessing a neural-immune circuit to control inflammation and shock. The Journal of Experimental Medicine 195, F25-F28.
Boulanger, L.M. (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64, 93-109.
Brenneman, D.E. et al. (1988) Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature 335, 639-642.
Bromley, S.K. et al. (2001) The immunological synapse. Annual Review of Immunology 19, 375-396.
Carr, D.J. et al. (1989) Hormones common to the neuroendocrine and immune systems. Drug Design and Delivery 4, 187-195.
Chacon, M.A. and Boulanger, L.M. (2013) MHC class I protein is expressed by neurons and neural progenitors in mid-gestation mouse brain. Molecular and Cellular Neuroscience 52, 117-127.
Chapman, R.C. et al. (2008) Pain and stress in a systems perspective; reciprocal neural, endocrine and immune interactions. The Journal of Pain 9, 122-145.
Cooper, E.L. (2010) Evolution of immune systems from self-not self to danger to artificial immune systems (AIS). Physics of Life Reviews 7, 55-78.
Elenkov, I.J. et al. (2000) The sympathetic nerve – an integrative interface between two super-systems; the brain and the immune system. Pharmacological Reviews 52, 595-638.
Goebel, M.U. et al. (2002) Behavioral conditioning of immunosuppression is possible in humans. FASEB Journal 16, 1869-1873.
Herbein, G. and Varin, A. (2010) The macrophage in HIV-1 infection; from activation to deactivation? Retrovirology 7, 33.
Malagoli, D. and Ottaviani, E. (2010) Life is a huge compromise; is the complexity of the vertebrate immune-neuroendocrine system an advantage or the price to pay? Comparative Biochemistry and Physiology, A 155, 134-138.
Ottavani, E. et al. (1988) The neuro-immunological interface in an evolutionary perspective; the dynamic relationship between effector and recognition systems. Frontiers in Bioscience 3, 431-435.
Ottovani, E. et al. (2007) Common evolutionary origin of the immune and neuroendocrine systems; from morphological and functional evidence to in silico approaches. Trends in Immunology 28, 497-502.
Ottaviani, E. and Franceschi, C. (1996) The neuroimmunology of stress from invertebrates to man. Progress in Neurobiology48, 421-40. Erratum in: Progress in Neurobiology 49, 285.
Pert, C. (1997) Molecules of Emotion; Why You Feel the Way You Feel. Schribner.
Pert, C. and Marriot, N. (2006) Everything You Need to Know to Feel Go(o)d. Hay House.
Polianova, M.T. et al. (2005) Chemokine receptor-5 (CCR5) is a receptor for the HIV entry inhibitor peptide T (DAPTA). Antiviral Research67, 83-92.
Pollitica, M. et al. (2007) Profound anti-HIV-1 activity of DAPTA in monocytes/macrophages and inhibition of CRR5-mediated apoptosis in neuronal cells. Antiviral Chemistry and Chemotherapy 18, 285-295.
Quan, N. and Banks, W.A. (2007) Brain-immune communication pathways. Brain, Behaviour and Immunity 21, 727-735.
Raberg, L. et al. (2002) Basal metabolic rate and the evolution of the adaptive immune system. Proceedings of The Royal Society of London, B 269, 817-821.
Rolff, J. (2007) Why did the acquired immune system of vertebrates evolve? Developmental and Comparative Immunology 31,476-482.
Rosi, S. et al. (2005) Chemokine receptor 5 antagonist D-ala-peptide T-amide reduces microglia and astrocyte activation within the hippocampus in a neuroinflammatory rat model of Alzheimer’s disease. Neuroscience 134, 671-676.
Ruff, M.R. et al. (2003) Update on D-ala-peptide T-amide (DAPTA); a viral entry inhibitor that blocks CRR5 chemokine receptors. Current HIV Research 1, 51-67.
Salles, A. et al. (2013) Barcoding T cell calcium response diversity with methods for automated and accurate analysis of cell signals (MAAACS). PLoS Computational Biology 9, e1003245.
Shatz, C.A. (2009) MHC class I: an unexpected role in neuronal plasticity. Neuron 64, 40-45.
Smith, E.M. and Blalock, J.E. (1988) A molecular basis for interactions between the immune and neuroendocrine systems. International Journal of Neuroscience38, 455-64.
Wahl, S.M. et al. (2006) HIV accomplices and adversaries in macrophage infection. Journal of Leukocyte Biology 80, 973-983.
Weigent, D.A. et al. (1990) Bidirectional communication between the neuroendocrine and immune systems. Common hormones and hormone receptors. Annals of the New York Academy of Sciences579, 17-27.
Wrona, D. (2005) Neural–immune interactions; an integrative view of the bidirectional relationship between the brain and the immune systems. Journal of Neuroimmunology 172, 38-58.
Yong, V.W. and Rivest, S. (2009) Taking advantage of the systemic immune system to cure brain diseases. Neuron 64, 55-60.
Yuan, S. et al. (2014) Amphioxus as a model for investigating evolution of the vertebrate immune system. Developmental & Comparative Immunology (in press).
Riddles in code; is there a gene for language?
‘I have…’
Words are like genes; on their own they are not very powerful. But apply them with others in the right phrase, at the right time and with the right emphasis, and they can change everything.
‘I have a dream…’
Genes are coded information. They are like the words of a language, and can be combined into a story which tells us who we are.
The stories we choose to tell are powerful; they can change who we become, and also change the people with whom we share them.
‘I have a dream today!’
Language is a means for coding and passing on information, but it is cultural, and definitely non-genetic. Nevertheless, for our speech capacity to have evolved, our ancestors must have had a body equipped to make speech sounds, along with the mental capacity to generate and process this language ‘behaviour’. Our body’s development is orchestrated through the actions of relevant genes. If the physical aspects of language ultimately have a genetic basis, this implies that speech must derive, at least in part, from the actions of our genes.
The hunt for genes involved with language led researchers at the University of Oxford to investigate an extended family (known as family KE). Some family members had problems with their speech. The pattern of their symptoms suggested that they inherited these difficulties as a ‘dominant’ character, and through a single gene locus.

The FOXP2 gene encodes for the ‘Forkhead-Box Protein-2’; a transcription factor. This is a type of protein that interacts with DNA (shown here as a pair of brown spiral ladders), and influences which genes are turne... mored on in the cell, and which remain silent. This diagram shows two Forkhead box proteins, which associate with each other when active. This bends the DNA strand and makes critical areas of the genetic code more accessible (Image: Wikimedia Commons)
Discovery of another unrelated patient with the same symptoms confirmed that the condition was linked to a gene known as FOXP2 (short for ‘Forkhead Box Protein-2’). This locus encodes a ‘transcription factor’; a protein that influences the activation of many other genes. FOXP2 was subsequently dubbed ‘the gene for language’. Is that correct?
Not really. FOXP2 affects a range of processes, not just speech. The mutation which inactivates the gene causes difficulties in controlling muscles of the face and tongue, problems with compiling words into sentences, and a reduced understanding of language. Neuroimaging studies showed that these patients have reduced nerve activity in the basal ganglia region of the brain. Their symptoms are similar to some of the problems seen in patients with debilitating diseases such as Parkinson’s and Broca’s Aphasia; these conditions also show impairment of the basal ganglia.

Genes code for proteins by using a 3-letter alphabet of adenine, thymine, guanine and cytosine (abbreviated to A, T, G and C). These nucletodes are knwn as ‘bases’ (are alkaline in solution) and make matched pairs w... morehich form the ‘rungs of the ladder’ of the DNA helix. Substituting one base for another (as happens in many mutations) can change the amino acid sequence of the protein a gene encodes. Changes may make no impact on survival, allowing the DNA sequence to alter over time. Changes that affect critical sections of the protein (e.g. an enzyme’s active site), or critical proteins like FOXP2, are rare (Image: Wikimedia Commons)
Genes provide the code to build proteins. Proteins are assembled from this coding template (the famous triplets) as a sequence of amino acids, strung together initially like the carriages of a train and then folded into their finished form. The amino acid sequences of the FOXP2 protein show very few differences across all vertebrate groups. This strong conservation of sequence suggests that this protein fulfils critical roles for these organisms. In mice, chimpanzees and birds, FOXP2 has been shown to be required for the healthy development of the brain and lungs. Reduced levels of the protein affect motor skills learning in mice and vocal imitation in song birds.
The human and chimpanzee forms of FOXP2 protein differ by only two amino acids. We also share one of these changes with bats. Not only that, but there is only one amino acid difference between FOXP2 from chimpanzees and mice. These differences might look trivial but they are probably significant. FOXP2 has evolved faster in bats than any other mammal, hinting at a possible role for this protein in echolocation.

Mouse brain slice, showing neurons from the somatosensory cortex (20X magnification) producing green fluorescent protein (GFP). Projections (dendrites) extend upwards towards the pial surface from the teardrop-shaped ce... morell bodies. Humanised Foxp2 in mice causes longer dendrites to form on specific brain nerve cells, lengthens the recovery time needed by some neurons after firing, and increases the readiness of these neurons to make new connections with other nerves (synaptic plasticity). The degree of synaptic plasticity indicates how efficiently neurons code and process information (Image: Wikimedia Commons)
Changing the form of mouse FOXP2 to include these two human-associated amino acids alters the pitch of these animals’ ultrasonic calls, and affects their degree of inquisitive behaviour. Differences also appear in their neural anatomy. Altering the number of working copies (the genetic ‘dose’) of FOXP2 in mice and birds affects the development of their basal ganglia.
Mice with ‘humanised’ FOXP2 protein show changes in their cortico-basal ganglia circuits along with altered exploratory behaviour and reduced levels of dopamine (a neurotransmitter that affects our emotional responses). So too, human patients with damage to the basal ganglia show reduced levels of initiative and motivation for tasks.
This suggests that FOXP2 is part of a general mechanism that affects our thinking, particularly around our initiative and mental flexibility. These are critical components of human creativity, and are as it happens, essential for our speech.
Basal ganglia circuits process and organise signals from other parts of the brain into sequences. Speaking involves coordinating a complex sequence of muscle actions in the mouth and throat, and synchronising these with the out-breath. We use these same muscles and anatomical structures to breathe, chew and swallow; our ability to coordinate them affects our speech, although this is not their primary role.
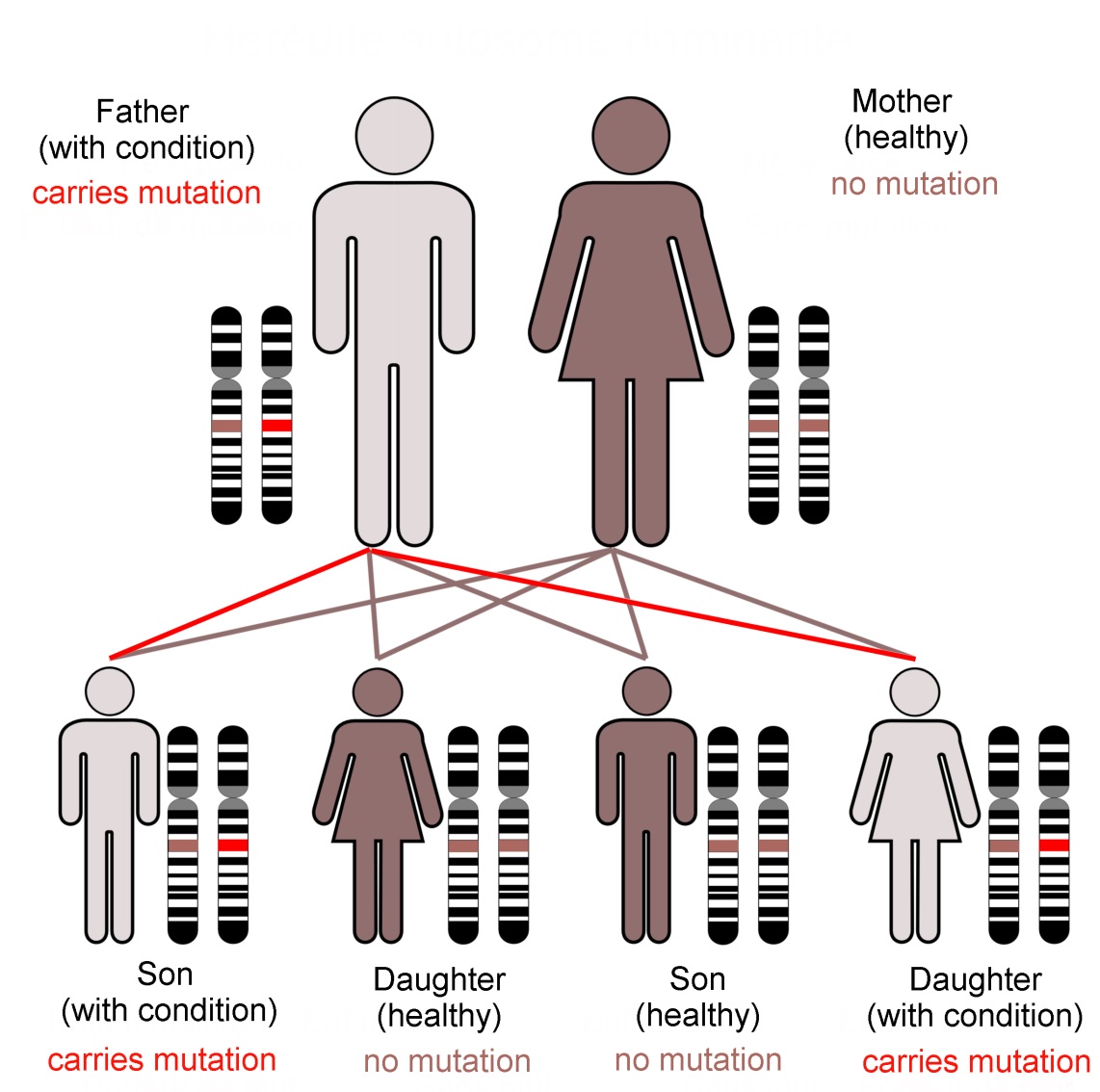
Family KE’s condition, caused by a dominant mutation in the FOXP2 gene, follows an autosomal (not sex-linked) pattern of inheritance, as shown here.Dominant mutations are visible when only one gene copy is present. In... more contrast a recessive trait is not seen in the organism unless both chromosomes of the pair carry the mutant form of the gene. The FOXP2 transcription factor protein is required in precise amounts for normal function of the brain. The loss of one working FOXP2 gene copy reduces this ‘dose’ which is enough to cause the problems that emerged as family KE’s symptoms (Image: Annotated from Wikimedia Commons)
In practice, very few of our 25,000 genes are individually responsible for noticeable characteristics. Most genetically inherited diseases result from the effects of multiple gene loci. FOXP2 is unusual because of its ‘dominant’ genetic character. It does not give us our language abilities, but it is involved in the neural basis of our mental flexibility and agility at controlling the muscles of our mouths, throats and fingers.
In addition, genes are only part of the story of our development. The way we think and subsequently behave alters our emotional state. Feeling stressed or calm affects which circuits are active in our brain. This alters the biochemical state of body organs and tissues, particularly of the immune system, modifying which genes they are using.
The dance between the code stored in our genes and the consequences of our thoughts builds us into what we are mentally, physically and socially. This story is ours to tell. By our experience, and with this genetic vocabulary, we create what we become.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Chial H (2008) ‘Rare genetic disorders: Learning about genetic disease through gene mapping, SNPs, and microarray data’ Nature Education 1(1):192 http://www.nature.com/scitable/topicpage/rare-genetic-disorders-learning-about-genetic-disease-979
Clovis YM et al. (2012) ‘Convergent repression of Foxp2 3′UTR by miR-9 and miR-132 in embryonic mouse neocortex: implications for radial migration of neurons’ Development 139, 3332-3342.
Enard, W (2011) ‘FOXP2 and the role of cortico-basal ganglia circuits in speech and language evolution’ Current Opinion in Neurobiology 21; 415–424
Enard, W et al (2009) A Humanized Version of Foxp2 Affects Cortico-Basal Ganglia Circuits in Mice Cell 137 (5); 961–971 http://www.sciencedirect.com/science/article/pii/S009286740900378X
Feuk L et at., Absence of a Paternally Inherited FOXP2 Gene in Developmental Verbal Dyspraxia, in The American Journal of Human Genetics, Vol. 79 November 2006, p.965-72.
Fisher SE and Scharff C (2009) ‘FOXP2 as a molecular window into speech and language’ Trends in Genetics 25 (4); 166-177
Lieberman P (2009) ‘FOXP2 and Human Cognition’ Cell 137; 800-803
Marcus GF & Fisher SE (2003) ‘FOXp2 in focus; what can genes tell us about speech and language?’ Trends in Cognitive Sciences 7(6); 257-262
Reimers-Kipping S et al. (2011) ‘Humanised Foxp2 specifically affects cortico-basal ganglia circuits’ Neuroscience 175; 75-84
Scharff C & Haesler S (2005) ‘An evolutionary perspective on Foxp2; strictly for the birds?’ Current opinion in Neurobiology 15:694-703
Vargha-Khadem F et al. (2005) ‘FOXP2 and the neuroanatomy of speech and language’ Nature Reviews Neuroscience 6, 131-138 http://www.nature.com/nrn/journal/v6/n2/full/nrn1605.html
Wapshott N (2013) ‘Martin Luther King's 'I Have A Dream' Speech Changed The World’ Huffington post, 28th August 2013 http://www.huffingtonpost.com/2013/08/28/i-have-a-dream-speech-world_n_3830409.html
Webb DM & Zhang J (2005) ‘Foxp2 in song learning birds and vocal learning mammals’ Journal of Heredity 96(3);212-216
The reptile that almost became a fish
‘It’s head is as long as I am tall!’
‘What is it?’
‘Hmm… A giant fish? A lizard? Don’t know.’
‘Well I know! It’s a sea dragon!’
It is spring, 1811, the morning after a storm. Mary is 12 years old, and fearless. She edges across the cliff to where her brother is already working to free the fossil bones. Fragments of weathered mudstone clatter down onto the beach. The eye sockets of the huge skull are wider than the span of her hand.

A 185 million year old fossil of Ichthyosaurus acutirostris beside ammonites (Harpoceras falcifer). This specimen shows the distinctive downward (hypoocercal) bend of the spine into the lower tail fluke, characteristic ... moreof this reptile group. The outlines of the fluked tail and dorsal fin are visible; these were supported by cartilage rather than bone as in modern fish. The huge eye sockets (relative to its body size) enabled these animals to hunt by sight for shellfish, small fish and squid in dimly lit or murky waters (Image: Wikimedia Commons)
During the 200 million years that dinosaurs roamed the land, the oceans were ruled by formerly land-dwelling reptiles. Of these, ichthyosaurs adopted dolphin-like forms, plesiosaurs became sea lion-like, and mosasaurs occupied crocodile-like ‘ambush’ predator roles.
Of these, ichthyosaurs were arguably the most successful. Their multiple adaptations to a fully aquatic life included huge eyes, a stiffened, fluked tail, and the ability to mate and give birth to live young in water. Much as killer whales do today, these predators structured the marine ecosystem. Many came to resemble the modern whales, dolphins and tuna fish that now fulfil similar ecological roles. The convergence of body forms between some ichthyosaur species and tuna are particularly astonishing because these reptiles were air breathers.
Why did icthyosaurs evolve to look like modern marine animals?

Ichthyosaurs first colonised the sea 250Ma ago. The earliest known aquatic ichthyosaur, the otter-like Utatsusaurus hataii (top) fed on fish and shellfish in shallow water. After the Cretaceous-Tertiary mass extinction ... more(65Ma), the land-based ancestors of modern whales also took to water. The early whale Kutchicetus minimus (bottom) had an otter-like ecological role, and converged to evolve a similar body form. Both had an undulating swimming style which was in the horizontal plane (like an eel) for Utatsusarus, and vertical for Kutchicetus. This reflects the style of locomotion inherited from their respective ancestors (Images: Wikimedia Commons)
Life in water poses a specific set of challenges. These marine reptiles fulfilled similar ecological roles to whales, and in time evolved body forms similar to these modern mammals. This is known as convergence.
Like whales and tuna, ichthyosaurs were adapted for long distance energy-efficient swimming. The respective horizontal and vertical strokes of both ichthyosaur and dolphin tails give an equally powerful ‘lift’ in both directions, propelling these animals forward in a near straight line.
A further example of this convergence is seen in the modern otter. Unlike the Ichthyosaurs, both whales and otters had land-based mammalian ancestors with a vertically moving spine, giving them their bounding gait. The whale-like ichthyosaurs moved their tail flukes horizontally, like a modern lizard.
Like whales and tuna, ichthyosaurs were adapted for long distance energy-efficient swimming. The respective horizontal and vertical strokes of both ichthyosaur and , propelling these animals forward in a near straight line.
What can modern animals tell us about ichthyosaurs?

The ichthyosaur Stenopterygius quadriscissus (above), became widespread, in the late Jurassic and early Cretaceous (160-100Ma). Its body shape is similar to that of the Bluefin tuna (Thunnus thynnus (below). Tuna hunt f... moreish and squid at around 500m depth. This is possible because they have a high oxygen intake, fast metabolic rate, warm muscles, an energy efficient swimming style, and a higher heart rate and blood pressure than other fish (Images: Wikimedia Commons)
Modern tuna and lamnid sharks have converged into a similar ‘deep water sprint-predator’ ecological role. These long distance migrants move constantly at a moderate speed, except for short fast bursts when chasing prey. Chunky vertebrae stiffen their bodies at their core, reducing sideways movements except at the narrow ‘hinge’ before the tail. The continuously active red ‘cruising’ muscles either side of the spine are warm, in marked contrast to most other fish. In combination with large tendons, these muscles work like pulleys, flicking the fluked tail from side to side. The surrounding white muscles give extra power during short ‘sprints’.
The ichthyosaur Stenopterygius was tuna-shaped with chunky stacked vertebrae. This stiffened body form suggests that these reptiles converged on the cruise-and-sprint deep water hunting role of modern tuna and lamnids.
Did ichthyosaurs have warm muscles like whales and tuna?

Cast of a skeleton of Hawkins’ plesiosaur (Thalassiodracon hawkinsi) from the Lower Lias strata at Street in Somerset; part of England’s Jurassic coast. These rocks are rich in marine fossils of all kinds including ... morefish, ammonites and belemnites (Image: Wikimedia Commons)
The isotopic proportion between the heavy 18O and the light 16O oxygen (the ratio is given as d18O) in the bones of living fish and marine animals decreases as body temperature increases. In principle we can use cold-blooded fish fossils as a ‘thermometer’ to indicate the water temperature, and compare this against isotope-predicted body temperatures for other fossil animals from the same rocks.
Oxygen isotope data allows us to infer that Jurassic plesiosaurs and ichthyosaurs had body temperatures of around 35⁰C, much higher than that of their environment. This indicates that they could generate heat, and may well regulated their body temperatures independently of their environment (homeothermy). Modern warm bodied marine animals have to conserve their body heat. This means it is reasonable to infer that ichthyosaurs used similar methods such as a counter-current blood circulation system and/or heat-insulating blubber.
What happened to the ichthyosaurs?

Plotosaurus bennisoni; a mosasaur from the Upper Cretaceous of North America. Most mososaurs lived in shallow coastal waters, although after the disappearance of the ichthyosaurs, some evolved into similar deep water sp... morerint predators. Plotosaurus had crescent-shaped tail flukes, equipping this animal to whale-like fast pursuit behaviour (Image: Wikimedia Commons)
Ichthyosaurs dominated the world’s oceans for around 150 million years, but then disappeared from the fossil record after the mid-Cretaceous (around 95Ma). The cause of their sudden extinction remains a mystery. The empty ecological roles that this created were later filled by mosasaurs; relatives of modern monitor lizards including the Komodo dragon. In turn these reptiles died out during the Cretaceous-Tertiary mass extinction (65Ma), making way for the later evolution of modern whales, dolphins and tuna.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Benson, R.B.J. and Butler, R.J. (2011) Uncovering the diversification history of marine tetrapods: ecology influences the effect of geological sampling biases In Comparing the Geological and Fossil Records: Implications for Biodiversity Studies ( A.J. McGowan and A.B.Smith, eds). Geological Society of London Special Publications 358, 191-208.
Bernal, D. et al. (2005) Mammal-like muscles power swimming in a cold-water shark. Nature 437: 1349-1352.
Brill, R.W. (1996) Selective advantages conferred by the high performance physiology of tunas, billfishes and dolphin fish. Comparative Biochemistry and Physiology, A 113, 3-15.
Brill, R.W. et al.(2005) Bigeye tuna (Thunnus obesus) behavior and physiology and their relevance to stock assessments and fishery biology. Collective Volume of Scientific Papers ICCAT 57, 142-161. Online;http://www.iccat.es/en/pubs_CVSP.htm
Dickson, K.A. and Graham, J.B. (2004) Evolution and consequences of endothermy in fishes. Physiological and Biochemical Zoology 77, 998-1018.
Donley, J.M. (2004) Convergent evolution in mechanical design of lamnid sharks and tuna. Nature 429, 61-65.
Fröbisch, N.B. et al. (2013) Macropredatory ichthyosaur from the middle Triassic and the origin of modern trophic networks. Proceedings of the National Academy of Sciences, USA 110, 1393-1397.
Graham, J.B. and Dickson, K.A. (2004) Tuna comparative physiology. Journal of Experimental Biology 297, 4015-4024.
Houssaye, A. (2013) Bone histology of aquatic reptiles: what does it tell us about secondary adaptation to an aquatic life? Biological Journal of the Linnean Society 108, 3-21.
Korsmeyer, K.E. et al. (1996) The aerobic capacity of tunas; adaptation for multiple metabolic demands. Comparative Biochemistry and Physiology, A 113, 17-24.
Lindgren, J. et al. (2007) A fishy mosasaur: the axial skeleton of Plotosaurus (Reptilia, Squamata) reassessed. Lethaia 40,153–160.
Lindgren, J. et al. (2011) Landlubbers to leviathans: evolution of swimming in mosasaurine mosasaurs. Paleobiology 37, 445-469.
Lingham-Soliar, T. and Plodowski, G. (2007) Taphonomic evidence for high-speed adapted fins in thunniform ichthyosaurs. Naturwissenschaften 94, 65-70.
Malte, H. et al. (2007) Differential heating and cooling rates in bigeye tuna (Thunnus obesus Lowe); a model of non-steady heat exchange. Journal of Experimental Biology 210, 2618-2626.
Motani, R. et al. (1998) Ichthyosaurian relationships illuminated by new primitive skeletons from Japan. Nature 393, 255-257.
Motani, R. (2002) Scaling effects in caudal fin propulsion and the speed of ichthyosaurs. Nature 415, 309-312.
Motani, R. (2002) Swimming speed estimation of extinct marine reptiles: energetic approach revisited. Palaeobiology 28, 251-262.
Motani, R. (2010) Warm-blooded “Sea Dragons”? Science 328, 1361-1362.
Rothschild, B. M. et al. (2012) Adaptations for marine habitat and the effect of Triassic and Jurassic predator pressure on development of decompression syndrome in ichthyosaurs. Naturwissenschaften 99, 443-448.
Syme, D.A. and Shadwick, R.E. (2011) Red muscle function in stiff bodied swimmers: there and almost back again. Philosophical Transactions of the Royal Society, B 366, 1507-1515.
Thewissen, J.G.M. and Bajpai, S. (2009) New skeletal material of Andrewsiphius and Kutchicetus, two Eocene cetaceans from India. Journal of Paleontology 83, 635-663.