Love in a time of Prozac; how did our emotions evolve?
Gut feelings; are microbes in your intestines dictating your mood?
A cat hears a scuffling sound amongst the garbage. She stands in shadow, all senses poised.
A few moments pass; the rat emerges. In this alleyway, a light breeze carries her scent towards him. He sniffs the air, and looks in her direction. Two eyes blink, and then fixate upon him.
But he does not heed the warnings. A parasite in his brain, picked up from cat faeces, has immobilised his fear response. He scuttles out across the floor…
We are mostly bacteria. The microbes living on our skin, on the inner surfaces of our lung linings and through our gut outnumber our body cells ten to one. In fact, bacteria and other micro-organisms live in and around all multicellular life forms – from plants to people. This ‘microbiome’ is
an essential part of our biology and our health, and even influences how we (and all other animals) behave.
Microbes begin to colonise our gut from the moment of our birth. They help to pre-digest our food, making nutrients soluble and hence available for absorption through the gut wall. But the helpfulness of these bacteria doesn’t stop here.
The many small fatty acids and amino acids produced by bacterial fermentation act as signals inside our bodies. They initiate the development of the network of nerves in the gut wall; our ‘enteric nervous system’ (also known as our ‘second brain’). These nerves control the rhythmical contractions of the digestive canal, which operate independently of the central nervous system. Bacterial products also initiate, nourish and maintain the cells lining our gut.

A thin section of the small intestine wall, stained for CK20 protein (found in the mucosal lining).The lining of our intestines is a vast sensory surface, whose cells are nourished by fatty acids, vitamins and other com... morepounds produced by bacterial fermentation. The finger-like projections (called villi) visible in this section, form our main absorptive surface for nutrients, and sense the presence of both friendly bacteria and harmful pathogens (Image: Wikimedia Commons)
However the role of this microbial community goes beyond digestion.
– They out-compete harmful bacteria, ‘policing’ the gut and maintaining its pH.
– They present antigen signals (bacterial surface proteins) to our immune system, training us to recognise ‘friend’ from ‘foe’.
– They produce neurotransmitters and hormones, which are used directly and indirectly by the body. These include the cytokines and chemokines needed by immune cells to induce inflammation and fever responses and to target white blood cells into infected tissues. Bacteria produce precursors for 95% of our body’s serotonin (the ‘feel good’ brain chemical), and 50% of our dopamine.
Bacterial products affect the formation of connections between neurons (the synapses).

The enteric nervous system interacts with the central nervous system via the vagus nerve (the parasympathetic system) and the prevertebral ganglia (sympathetic nervous system). However if these nerve connections are sev... moreered, the enteric system will continue to function, integrating and resolving signals from the body and the environment. This network uses around 30 neurotransmitters, most of which are also found in the brain, and which include acetylcholine, dopamine and serotonin (Image: Wikimedia Commons)
Our ‘gut-brain axis’ is linked indirectly by chemicals the bacteria produce, which activate the immune system. Our mind and gut is also linked directly via the vagus nerve.
The vagus or ‘wandering’ nerve, our 10th cranial nerve, forms part of the parasympathetic (involuntary) nervous system. It is a ‘mixed nerve’, meaning it carries both sensory information about our body state back to the brain, including from the gut to the brain stem, and relaying messages from the brainstem and emotional centres to the body.
This information highway operates the ‘vagal reflex’, which relaxes the muscles around the stomach wall making space for our food, and also integrates the digestive process with our blood circulation, hormone system and emotional state.
This conversation between the digestive, immune, hormonal and nervous systems is essential for our healthy development. Mice raised under sterile conditions (so that their guts are free of bacteria) develop fewer connections between neurons, which results in retarded brain growth.
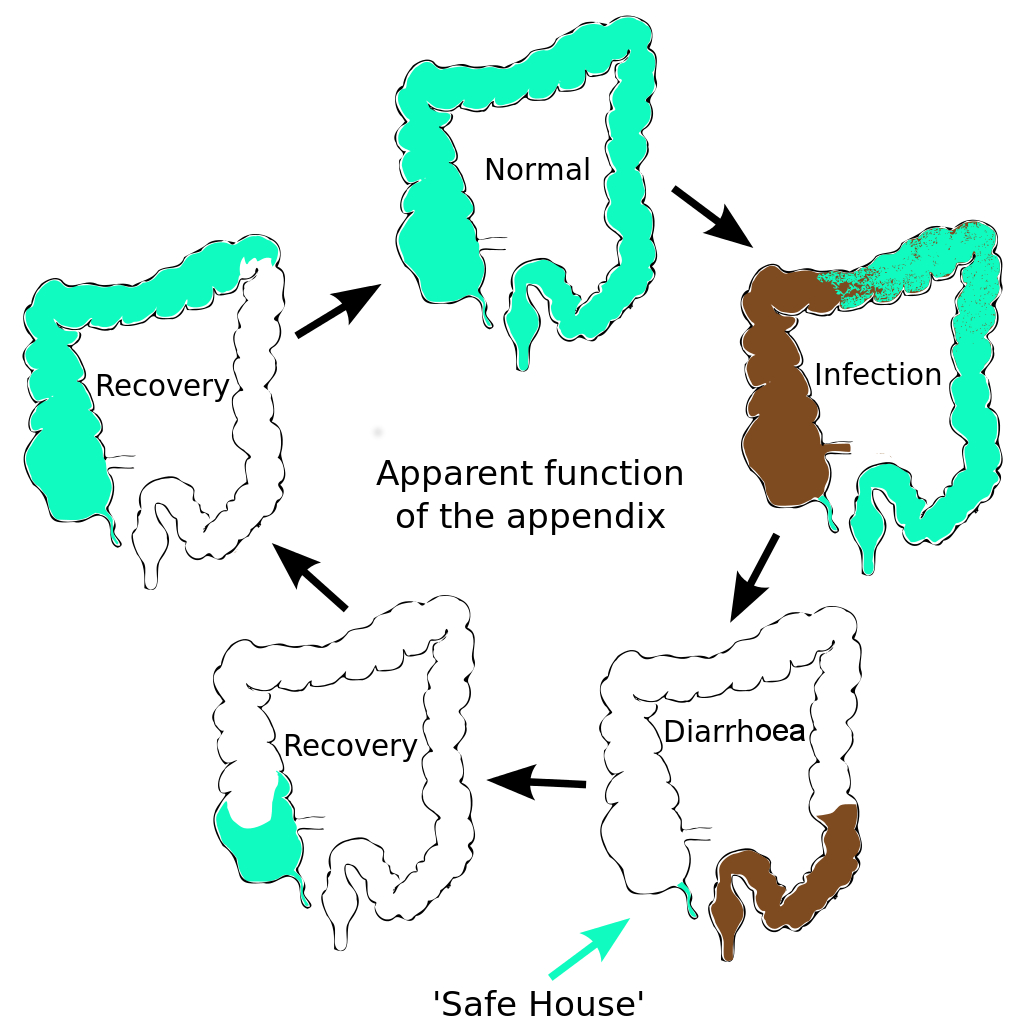
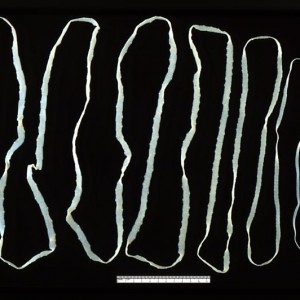
The human appendix is surrounded by copious amounts of immune tissue which particularly nurture and protect this sub-sample of our gut bacterial community. This creates a ‘safe house’ for a sample of our gut microfl... moreora. After an infection has triggered a diarrhoea response which expels the intestinal contents, the guts are recolonized by bacteria from this reservoir in the appendix. Charles Darwin first suggested that the human appendix is a relic of our evolution; a ‘vestige’ of a much larger mammalian caecum. (A caecum is a larger area of gut, containing bacteria, adapted in herbivores to digest large amounts of plant material). However this explanation doesn’t fit the facts. The appendix has evolved at least twice, arising independently in marsupials and placental mammals; an example of evolutionary convergence. This suggests that it has a current and selectable function (Image: Amended from Wikimedia Commons)
Gut microbes also influence our mood and emotions, affecting our behaviour. If intestinal bacteria from timid mice are used to populate the guts of a normally inquisitive mouse strain, these more adventurous mice become timid. Likewise, timid mice become adventurous when given the microflora from inquisitive mice.
Behavioural effects are also visible in humans. One of the first studies, conducted in France by Michaël Messaoudi and co-workers, found that drinking milk fermented with probiotic bacteria (versus non-fermented milk) lifted the mood of healthy human volunteers, reduced their blood cortisol levels (a hormone which increases during stress) and gave them a greater resilience against stimuli provoking symptoms of depression and anxiety.
Just as gut bacteria influence our health and mood, experiences that change our mood and behaviour in turn affect the composition of this microbial community. This shows that our gut microbiota are not autonomous, but act like a fully integrated body organ. This microbiotic organ monitors and responds to the food we eat, influences how we respond to our world, and connects with our body using messages sent in a common chemical ‘language’ . We speak about our ‘gut feelings’, usually unaware that this is much more than a metaphor.
It seems then, that our thoughts, feelings and behavioural choices affect our gut microbiome. As we ‘tell them’ how we feel (through the parasympathetic nervous system, immune system and hormones), they align their metabolic responses with this information. Their biochemical cues reinforce the body’s signal by releasing neurologically active chemicals that affect our mood. If our enteric nervous system really does deserve its title of the ‘second brain’, then the bacteria in our gut are mediating a connection that integrates the ‘thoughts of our guts’ with those of our mind.

Lab mouse, strain mg 3204. Laboratory mice are usually derived from the house mouse (Mus musculus). Mice are useful as a system to study many aspects of human health, thanks to having a high similarity with our genetic ... morecode, as well as the ability to thrive in a human-influenced environment. As with humans, many aspects of their development and behaviour are dependent upon a healthy community of gut microflora (Image: Wikimedia Commons)
Since we and all other animals are intimately associated with our gut bacterial ‘organ’, this raises interesting questions at the cellular level about where our physical boundaries really are.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Collins, S.M. and Bercik, P. (2013) Intestinal bacteria influence brain activity in healthy humans. Nature Reviews Gastroenterology & Herpetology 10, 326-327.
Cryan, J.F. and Dinan, T.G. (2012) Mind-altering micro-organisms; the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience 13, 701-712.
David, L.A. et al. (2014) Diet rapidly and reproducibly alters the gut microbiome. Nature 505, 559-566.
Diamond, B. et al. (2011) It takes guts to grow a brain. Bioessays 33, 588-591.
Faith, J.J. et al. (2011) Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science 333, 101-104.
Forsythe, P. et al. (2010) Mood and gut feelings. Brain, Behaviour and Immunity 24, 9-16.
Frank, D.N. and Pace, N.R. (2008) Gastrointestinal microbiology enters the metagenomics era. Current Opinion in Gastroenterology 24, 4-10.
Heijtz, R.D. et al. (2011) Normal gut microbiota modulates brain development and behaviour. Proceedings of the National Academy of Sciences, USA 108, 3047–3052.
Jahan-Mihan, A. (2011) Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract. Nutrients 3, 574-603.
Kurokawa. K. et al. (2007) Comparative metagenomics reveals commonly enriched gene sets in human gut microbiomes. DNA Research 14, 169-181.
Lee, W.J. and Brey, P.T. (2013) How microbes influence metazoan development: insights from history and Drosophila modelling of gut-microbe interactions. Annual Review of Cell and Developmental Biology 29, 571-592.
Lee, W.J. and Hase, K. (2014) Gut microbiota-generated metabolites in animal health and disease. Nature Chemical Biology 10, 416-424.
Lyer, L.M. et al. (2004) Evolution of cell-cell signalling in animals: did late horizontal gene transfer from bacteria have a role? Trends in Genetics 20, 292-299.
Messaoudi, M.et al. (2011) Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition 105,755-764.
Montiel-Castro, A.J. et al. (2013) The microbiota-gut-brain axis: neurobehavioural correlates, health and sociality. Frontiers in Integrative Neuroscience 7, article 70.
O’Hara, A.M. and Shanahan, F. (2006) The gut flora as a forgotten organ. EMBO Reports 7, 688-693.
Randal Bollinger, R. et al. (2007) Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. Journal of Theoretical Biology 249, 826-831.
A sense of protection: is the immune system an extension of our minds?
Entering a body is much like entering a country.
At the border, agents verify the identity of those seeking to pass, and establish their status as ‘native’, a ‘naturalised resident’, or ‘alien’. Clarity about the identity of these travellers allows them to be appraised as ‘friend’ or ‘foe’.
The body of a nation operates best with safety measures in place. Its borders are a first line of surveillance that ascertains when to mobilise defences.
Border checks in some countries are more efficient; they deploy new technologies to implement higher orders of monitoring and identification.

Scanning electron microscope image of a neutrophil, a cell from the innate immune system (yellow), engulfs anthrax bacteria (orange); scale bar = 5 micrometres.Body cells that experience stress, wounding, or sense the p... moreresence of bacteria, produce cytokines and other signals that trigger the innate immune response, and attract immune cells into the tissues.Some of these cells (‘phagocytes’) like this neutrophil, engulf and digest the invaders, whilst others (‘granulocytes’) secrete granules of cytokine, histamine and various types of oxygen free radicals, all of which enhance the inflammation response. Proteins in the blood, the ‘compliment system’, also condense onto and coat unwanted bacterial invaders and other particles. This targets them for destruction by phagocytes, or removal from the blood stream in the spleen (Image: Wikimedia Commons)
Typically we think of the immune system as a defence mechanism; the forces we mobilise to fight disease. Animals, plants, fungi and microbes all have a form of this type of defence. Even bacteria have a version of immunity, using enzymes that can react to and then digest viral proteins.
Immune responses in animal systems are thought of as having two levels of action.
Innate immunity
First, ‘innate immunity’ provides a spectrum of responses which use secreted proteins and dedicated cell types to target common components of a broad range of diseases.
Amongst animals, invertebrate bodies are relatively simple. In contrast, vertebrates have increased genetic and physical complexity, more sophisticated sensory perceptions and cognitive processing and, in some clades, enhanced social organisation and communication. This increased complexity is reflected in the vertebrate immune system.
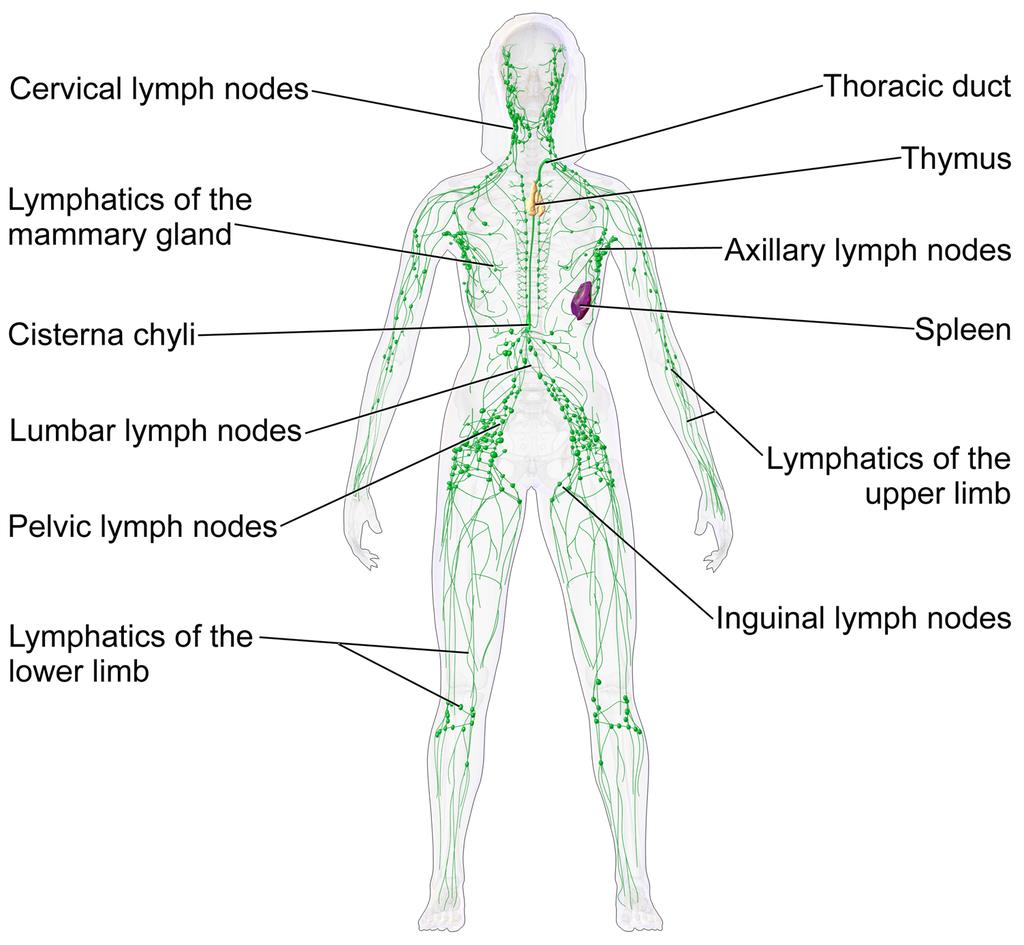
The lymphatic system of a human female. This network of vessels allows immune system cells rapid access to all areas of the body. Lymph vessels interconnect with the blood circulation at the heart and lymph nodes.Two or... moregans are particularly important in this network.• The thymus gland polices antibody-producing adaptive immune system cells, filtering out those which are too close to recognising native body proteins.• The spleen synthesises antibodies in its white pulp, and releases these proteins into the blood stream, where they bind to foreign proteins, such as those present on the surface of bacteria. This targets cells and other debris in the blood. The spleen removes antibody-coated bacteria and old red blood cells from the circulation. It also acts as a reservoir for monocytes, immune cells which later develop into phagocytic (non-specific engulfing) cells (Image: Wikimedia Commons)
Adaptive Immunity
The second form of immune response, known as ‘adaptive immunity’, refers to the ability to identify and target specific invaders. In vertebrates, this immune function first appeared in jawed fish.
In broad terms, it involves three phenomena.
– A new immunological organ (the thymus).
– A repertoire of specialised lymphocyte cells including T-cells (matured in and released from the thymus gland) and B-cells (released from the bone marrow), some of which produce antibodies.
– The production of a ‘self-identity’ signature, the ‘Major Histocompatibility Complex’ (MHC) proteins, in all body cells.
The community of bacteria associated with our gut, lungs and other surfaces comprise the first line of defence. This community of microbes (our ‘microbiome’) out-competes many harmful agents (pathogens).

Like the gut, our lung linings have a microbiome. Both ’friendly’ microbes and infective agents (here Streptococcus pneumoniae and Pseudomonas aeruginosa) produce proteins and other chemicals that act as signals. C... moreells lining our lungs act as signal receivers and recognise these chemicals, producing cytokines and other cues which relay messages to the immune system.(Image: Wikimedia Commons)
If our body cells become infected or damaged, they release stress signals such as oxygen and nitrogen free radicals and various peptides (short strings of amino acids) such as cytokines and defensins. These cues act as messages to the immune system, potentially triggering inflammation and other chemical defences which attract immune cells into the tissue by chemotaxis.
The microbial community also produces these immune-stimulating signals, along with brain-active chemicals (neurotransmitters). In addition, these signals are also generated, recognised by, and responded to by immune system cells.

Diagram of an antibody. These immune system proteins are a complex of four separate protein chains. In this diagram, blue regions are consistent whilst the yellow regions are variable. They are secreted into the blood s... moretream or gut. Adaptive immune cells use them as ‘antennae’. When this signal receiver encounters an antigen that is a good fit, this triggers an adaptive response. All proteins interact by shape. Antibodies have a recognition region which is highly variable. The strongest immune reactions provide a ‘best fit’ to this three-dimensional molecular jigsaw. (Image: Wikimedia Commons)
Our neurons and immune cells both respond to hormones within our bodies, since chemically these are close to neurotransmitters. This means that our brain, hormonal system, sensory nerves and extended microbial community all share a common chemical language with the innate immune system, present in all animals.
The greater the biochemical diversity of cell populations in a body, the more opportunities there are for bacteria or viruses to find novel ways of attacking these cells. For this reason, adaptive immune system cells themselves are vulnerable to certain types of disease. For instance AIDS (Acquired Immuno-Deficiency Syndrome), caused by the Human Immuno-deficiency Virus (HIV), specifically targets and infects the adaptive immune system’s T-cells, using the very mechanism that allows these cells to detect infection – their surface antibodies.
Potentially then, adaptive immunity provides a much more sophisticated and versatile immune mechanism. For vertebrates with their adaptive system to be ultimately more vulnerable to certain infections than the innate-only immunity of invertebrates seems bizarre and inefficient. Yet adaptive immune systems have evolved independently twice in vertebrates. This suggests that the adaptive mechanism must convey some survival advantages, but disease resistance may not be the primary role which has driven its evolution.
How does the system work, and what is the real purpose of adaptive immunity?
Adaptive mechanisms give our immune system a ‘memory’
In vertebrates, ‘adaptive immunity’ adds an additional higher-order function to the innate immune system by providing a means to both recognise and ‘remember’ specific diseases. To do this, they use a protein recognition system; these are the familiar antibodies.
These proteins have a shape-specific region which fits the shape of part of a foreign protein (an antigen) like a key in a lock. An antigen is a molecule that is capable of triggering an immune response; this can be from a foreign source (e.g. a virus) or produced by an unhealthy body cell, e.g. a cancer cell.

The adaptive immune system “adapts” to infections that get past our innate defences. Phagocytic macrophage (innate system) cells behave like amoebae, and can engulf and digest foreign bacteria. These ce... morells become ‘antigen presenting cells’ (APC), and displaying short pieces of bacterial protein (the antigen) on their cell surfaces, along with a self-signal, the ‘Major Histocompatibility Complex’ class II protein (MHC2). The MHC tells the immune system that this cell is a messenger, not an invader. These two proteins together activate the adaptive immune system.There are two types of adaptive response. First, T lymphocytes (T cells) display an antibody on their surface that recognises the foreign fragment on an APC. They then produce other surface proteins (here CD4+) that signal a change of identity, turning them into ‘‘helper’ cells. Helpers can provoke macrophages or B lymphocytes (B cells) to act. An activated B cell releases antibodies into the blood stream that ‘seek out’ and identify the invader. T cells can also become ‘killers’ that recognise and destroy infected body cells. (Image: Wikimedia Commons)
Adaptive immune cells called T-cells produce antibodies and ‘display’ them from their cell surfaces, using them as ‘antennae’ to detect invaders. Phagocytic (engulfing) innate immune cells can act as antigen presenting cells, displaying foreign protein fragments (antigens) on their surfaces. T-cells whose antibody ‘fits to’ this antigen can then be triggered to respond.
Triggered T-cells divide multiple times, so producing identical clones of themselves. This increases the magnitude of the body’s immune response to the infection. Activated T-cells relay the signal in turn to adaptive B-cells, triggering them to secrete large amounts of antibody proteins specific to this foreign antigen into the blood. These bind to the foreign antigens, coating the virus particles or foreign bacteria in antibodies. This targets the innate system’s macrophages to ‘engulf and destroy’.
Immune system cells interact with each other in a similar way to nerve cells
The association between the antigen presenting cell and the activating T-cell is very close, and the trigger mechanism transmits a one-way signal between the cells. This is very like how a nerve synapse transmits a signal to the next nerve. It is therefore entirely reasonable to call it an ‘immunological synapse’.
This between-cell interaction, which here involves a specific recognition between the immune cells, arises from an ancient, general mechanism of between-cell communication that allows signal-transmitting contacts to form. Activated T-cells initially show pulses of electrical activity, produced by a release of calcium ions (which carry an electrostatic charge) into the cell matrix (cytoplasm).
Synapses are stable contacts that form between two distinct cells that allow for information transfer through directed secretion. Synapses between neurons in the brain form relatively stable contacts, although these remain ‘plastic’, able to be recruited into new neural networks and adopted for new purposes.
In the immune system, ‘synapse’ formation between for example an antigen presenting cell and a T-cell, provokes the T-cell to initiate a signal relay transmitted to other cells which produces a range of specific ‘actions’. These responses, resulting in defences that target the specific foreign antigen, are produced by cells further downstream of this initial ‘synapse’.

When a patient is first vaccinated, the adaptive immune system raises a response against the foreign antigens. These cells persist at a high level for several weeks, then the antibody level and effector T-cell activity ... moregradually declines. When a patient is re-vaccinated, a stronger response results. Selection favours T-cells with small differences in antibody binding that give a stronger reaction. These highly specific clones remain in the serum and lymphatic tissues and provide protective immunity against reinfection by the same agent. This gives a longer term immunological memory; later reinfection leads to a rapid increase in antibody production and effector T cell activity, which often prevents the disease from being apparent (Image: Wikimedia Commons)
Vaccinations activate our body ‘memory’
By cloning and maintaining a population of cells with disease-specific antibodies, our immune system ‘remembers’ how it responded to the disease. A second exposure produces a refinement of the accuracy of the recognition mechanism, and raises a population of ‘memory T-cells’ that persist in the body for much longer. This is the basis of vaccination. Exposing our immune systems to proteins from the infective agent, via an injection, allows us to develop resistance in a controlled, safe way. Our bodies raise a population of immune cells that ‘remember’ how to recognise these foreign proteins. If we later meet the disease, these ‘memory cells’ activate, enabling us to more quickly overcome the infection and contract only a mild version of the disease, or perhaps experience no symptoms at all.
These mechanisms suggest that adaptive immunity should provide enhanced resistance to diseases beyond what the innate response alone can deliver. In practice, however, invertebrates seem to be vulnerable to fewer diseases than we are. This may be a result of their simpler and less diverse profile of cell and tissue types.

Diagram of an HIV particle. Using cell surface antibodies as an infective agent identification mechanism means that immune cells can in turn become ‘visible’ to these pathogens. The Human Immunodeficiency Virus (HIV... more) which causes Acquired Immuno-defficiency Syndrome (AIDS) specifically identifies T-cells. This virus is a master of disguise; it uses a similar gene shuffling mechanism as are used to produce variable proteins on the surface of its viral capsule. This changeability makes the virus seem to always be something new – this is the reason that the immune system has difficulty recognising and neutralising it, and also is why the virus has been so difficult to target using a vaccine. The interaction between an infection and the host immune system is usually like a predator prey relationship. The immune system is the ‘predator’, chasing down to consume the infection (the ‘prey). In the case of HIV, these roles are unclear. Is the virus pursing the immune cell, or is the immune cell pursuing the virus? (Image: Wikimedia Commons)
Why would vertebrates evolve this expanded complexity in their immune system?
Every living thing, including plants and bacteria, possess an immune system of some kind. What we recognise as innate immunity is found in plants and also throughout the animal kingdom. Adaptive immunity raises the degree of complexity. Significantly, it has evolved twice independently within the vertebrates; in jawed vertebrates (sharks to humans) and also in the more ‘primitive’ jawless clades. Certain invertebrate groups (e.g. snails and insects), and indeed also bacteria, have a quasi-adaptive system, using specific, cross-reactive molecules, although these systems are not as sophisticated as that found in the vertebrates.

A river lamprey (Lampetra fluviatilis) from the German North Sea. The immune systems of this and other jawless fish such as the hagfish have been found to contain adaptive cells which act in a similar way to T- and B- l... moreymphocytes of the jawed vertebrates. Here the membrane proteins acting as signal receivers are different, based on receptors containing leucine-rich motifs. As in their jawed cousins, the immune responses of lampreys produce a clonal increase of specific disease-detecting cells (Image: Wikimedia Commons)
Studies in the jawless fish, lamprey and hagfish, show that a very different form of adaptive system has arisen than is found in jawed vertebrates, co-opted from a different set of genes and proteins. In contrast, their immune molecules include proteins with repeated sections in the chain which have multiple residues of the amino acid leucine (termed ‘Leucine Rich Repeats’, or LRRs). A form of LRR-based adaptive immunity is also found in plants.
Jawed vertebrate adaptive immunity however is rather more developed than that of the jawless fish. Jens Rolff makes two intriguing suggestions as to what may have driven its evolution.
i. Vertebrate bodies are larger and more complex than invertebrates, providing a new set of potential habitats for parasites. Rolf suggests that an evolutionary ‘arms race’ may have occurred between vertebrates and a group of parasitic Platyhelminthes, which include tapeworms and liver flukes. These parasites began to diversify after the evolutionary divergence between jawed and jawless fish.
The surface of these parasites is highly resistant to our immune defences, suggesting that they co-evolved in tandem with an immune-competent host. The increasingly more aggressive and targeted immune response required from the host to expel these parasites may have selected for the direct recognition mechanism and targeted responses of adaptive immunity. This theory also implies that the adaptive components of the immune system originally evolved in association with the gut.
ii. Vertebrates have higher metabolic rates than invertebrates. This allows for greater rates of activity and access to a wider range of ecological habitats. Higher energy bodies require more sophisticated brains and nervous systems, and have higher maintenance demands.

Scheme showing a DNA ligase-I enzyme (in color) repairing a break in DNA. DNA damage, caused by environmental factors and normal metabolic processes, occurs at a rate of 1,000 to 1,000,000 molecular lesions per cell per... more day. Without maintenance to mend such breaks, cells can malfunction, die, or become cancerous. Ligases catalyse the crucial step of joining the breaks in the spiralling DNA strand. To do so they require energy, in the form of either Adenosine triphosphate (ATP) or Nicotinamide adenine dinucleotide (NAD+) as a cofactor (Image: Wikimedia Commons)
Rolff suggests that the adaptive immune system is part of the normal homeostasis of a higher energy metabolism. Such regimes also produce higher concentrations of oxygen free radicals and other stress-associated compounds which can damage DNA and cause cancers. High energy vertebrate bodies therefore require a means of self-monitoring. The adaptive immune system may provide just such a set of ‘internal eyes and ears’.
How does the adaptive immune system function in vertebrates?
Our immune system discerns ‘friend’ from ‘foe’, ‘self’ from ‘non-self’ and monitors the status and identity of our own body cells. The adaptive system provides specific ways to identify and monitor foreign and native body cells.
An immunological synapse relay is triggered when for example a T-cell antibody recognises a foreign protein on an ‘antigen presenting cell’. This and other types of immunological synapse require a class of proteins related to antibodies; the ‘Major Histocompatibility Complex’ (MHC) proteins.
Vertebrates produce and display MHC proteins on their cell surfaces. During an infection, if a virus or microbial pathogen enters a body cell, it becomes invisible to the immune system. Infection, however, alters the health of the cell, reducing its surface MHC production. Reduced or absent MHC triggers ‘Natural Killer’ (NK) cells to form an immunological synapse with them, this time triggering the destruction of the infected body cell, and helping to reduce the spread of the disease.

As a cell is first infected by a microbial pathogen, (as in this diagram), it quickly mobilises its protein-dismantling enzymes (the ‘proteasome’) to digest the foreign proteins into short fragments (peptides). Spec... moreialised pores (‘Transport of Alien Protein’ or TAP channels) capture the fragments and shunt them into the endoplasmic reticulum, where the cell assembles its own proteins. Here the fragments are bound to ‘Major Histocompatibility Complex’ class 1 (MHC I) proteins. These are transported to the cell surface where they protrude like a beacon, presenting the foreign protein to the innate immune system’s ‘Natural Killer’ cells (Image: Wikimedia Commons)
MHC proteins also act as antigen presenting molecules. At an early stage of infection, the cell’s own defences may digest some of the invading pathogen’s proteins. These foreign antigens can be bound by MHC molecules, and presented on the cell surface. Again, this triggers NK cells to target this body cell for destruction.
Antigen-presenting phagocytic immune cells also do this using a different class of MHC protein, which avoids them activating the NK cells.
Recent research has also shown an unexpected role for MHC proteins in neuronal plasticity. Brain neurons require MHC class I molecules to establish new neural networks. Along with a range of other immune system receptors, these molecules are critical for the construction of new connections and the remodelling of synapses. This suggests that the transmission of a signal across the neural synapse and the immunological synapse are likely using the same mechanism.
What does this suggest as the primary role of adaptive immunity?

The ‘molecules of emotion’ with their effects. The mood effects that result from their deficiency in italics. These neurotransmitters are the same chemical language as is used by cells of the immune system (Image: W... moreikimedia Commons)
Candace Pert’s work demonstrated that immune cells both respond to and produce the same neurotransmitter signals as brain neurons. She initially found opiate receptors from the brain in immune system cells. Opiates are drugs that trigger the body’s responses to endorphins, the body’s natural ‘feel good’ chemicals. Pert showed that receptors for neurotransmitters which affect our mood; the ‘molecules of emotion’ including b -endorphins and serotonin, are present in immune system cells. Immune cells also manufacture these same chemicals.
Immune cells are everywhere in the body. Brain-based phenomena such as depression impact the function of the immune system such that our level of disease resistance rises and falls with our moods. In at least chemical terms, these cells are operating in conjunction with our neural network. Pert’s results reveal that through the immune system, our minds occupy the full sensory space of our bodies, and are not limited to our brains. By the same route, immune cells convey the chemical implications of our emotional state into every cell in our body. This means that the way we think has a direct impact on our health.
Adaptive immune cells recognise stress signals, neurotransmitters, and foreign proteins. Foreign antigen recognition activates the immunological synapse, and triggers the adaptive immune response. Recent research has shown that the MHC proteins, essential for this response, are also involved in the functioning of nerve synapses in the brain. This implies that the two systems are really both part of the same ‘neural super-organ’.
This ‘organ’ has flexibility as to where it focusses its attention. Release of cytokines and other stress signals draw immune cells into tissues where their surveillance is most required. This roving internal sense organ has been referred to by Enzo Ottavani and Claudio Franceschi as an ‘immune-mobile brain’ whose ‘eyes and ears’ are our adaptive immune cells.

Poster, emphasising the importance of surveillance, issued by the office for Emergency Management in the United Kingdom during the Second World War (Image: Wikimedia Commons)
This surveillance system cross-reacts with and can mutually influence the body’s hormonal system and gut microbiome. Edwin Blalock has suggests a primary role for the immune system as a sense organ; truly our ‘sixth sense’, which is able to detect stimuli not recognised by the central or peripheral nervous system. This information, the chemical language of the body’s bacterial community, is translated into a cognitively accessible form by the antennae of the adaptive immune system.
This provides a picture of the brain and immune system as forming an integrated ‘body-mind’. This system’s sensory perceptions, thoughts, emotions and behavioural responses impact the physical body at the biochemical level. This ‘embodiment’ idea has also become apparent through recent insights into the actions of mirror neurons and the neural basis of human language.
Having a conversation in the same language synchronises our minds. The brain and immune system translate this into hormonal and other chemical cues of emotion.
This suggests that our immune system is one part – a subconscious part – of our overall consciousness. This consciousness is integrated into our ‘body-mind’. Through sharing our thoughts with others, our awareness operates beyond the body at a higher level, and as we do this, we connect at the level of our chemistry.
Conclusions
- Innate immunity is general, and present in all cells in some form, from bacteria to humans.
- Adaptive immunity, where the organism raises a specifically cross-reacting chemical response to an infection, has evolved multiple times in multicellular life forms, including twice in vertebrates. Characteristics of adaptive immune systems in vertebrates are the appearance of cross-reacting proteins (antibodies) produced by specialised immune cells, a self-identifier (the major histocompatibility complex) in all body cells, and a thymus gland, which is a new organ within the lymphatic system.
- The driver of immune system evolution in vertebrates is not primarily defence. Factors that have driven the evolution of immune complexity in the vertebrate system (relative to invertebrates) may include (i) evolutionary ‘arms races’ with gut parasites, and (ii) an increased need to monitor the metabolic by-products of the higher energy vertebrate body.
- Immune complexity seems to be linked to the complexity of the overall organism, and fulfils a self-surveillance role. The increased complexity of vertebrate systems has in fact made them vulnerable to a wider range of infections than invertebrates.
- Vertebrate immune cells, neurons, and body cells all share a common chemical language; a language also used by the associated microbes that live on and around the vertebrate body (the microbiota).
- Immune cells form synapses, using a similar between-cell communication apparatus that neurons use to form their synapses. However the immune synapses are more transient, and this component of the body’s relay network is able to migrate to tissues where it is needed, augmenting the body’s surveillance in this area.
- Brain-body communication is two-way. The immune system integrates our thoughts and emotional state with our body, as well as informing the mind of the status of the body’s cells and tissues.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Abedon, S.T. (2012) Bacterial ‘immunity’ against bacteriophages. Bacteriophage 2, 50–54.
Bellinger, D.L. and Lorton, D. (2014) Autonomic regulation of cellular immune function. Autonomic Neuroscience 182, 15-41.
Bhalla, A.K. (1989) Hormones and the immune response. Annals of the Rheumatic Diseases 48, 1-6.
Bhat, R. and Steinman, L. (2009) Innate and adaptive autoimmunity directed to the central nervous system. Neuron 64, 123-132.
Blalock, J.E. (2005) The immune system as the Sixth Sense. Journal of Internal Medicine 257, 126-138.
Blalock, J.E. (2002) Harnessing a neural-immune circuit to control inflammation and shock. The Journal of Experimental Medicine 195, F25-F28.
Boulanger, L.M. (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64, 93-109.
Brenneman, D.E. et al. (1988) Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature 335, 639-642.
Bromley, S.K. et al. (2001) The immunological synapse. Annual Review of Immunology 19, 375-396.
Carr, D.J. et al. (1989) Hormones common to the neuroendocrine and immune systems. Drug Design and Delivery 4, 187-195.
Chacon, M.A. and Boulanger, L.M. (2013) MHC class I protein is expressed by neurons and neural progenitors in mid-gestation mouse brain. Molecular and Cellular Neuroscience 52, 117-127.
Chapman, R.C. et al. (2008) Pain and stress in a systems perspective; reciprocal neural, endocrine and immune interactions. The Journal of Pain 9, 122-145.
Cooper, E.L. (2010) Evolution of immune systems from self-not self to danger to artificial immune systems (AIS). Physics of Life Reviews 7, 55-78.
Elenkov, I.J. et al. (2000) The sympathetic nerve – an integrative interface between two super-systems; the brain and the immune system. Pharmacological Reviews 52, 595-638.
Goebel, M.U. et al. (2002) Behavioral conditioning of immunosuppression is possible in humans. FASEB Journal 16, 1869-1873.
Herbein, G. and Varin, A. (2010) The macrophage in HIV-1 infection; from activation to deactivation? Retrovirology 7, 33.
Malagoli, D. and Ottaviani, E. (2010) Life is a huge compromise; is the complexity of the vertebrate immune-neuroendocrine system an advantage or the price to pay? Comparative Biochemistry and Physiology, A 155, 134-138.
Ottavani, E. et al. (1988) The neuro-immunological interface in an evolutionary perspective; the dynamic relationship between effector and recognition systems. Frontiers in Bioscience 3, 431-435.
Ottovani, E. et al. (2007) Common evolutionary origin of the immune and neuroendocrine systems; from morphological and functional evidence to in silico approaches. Trends in Immunology 28, 497-502.
Ottaviani, E. and Franceschi, C. (1996) The neuroimmunology of stress from invertebrates to man. Progress in Neurobiology48, 421-40. Erratum in: Progress in Neurobiology 49, 285.
Pert, C. (1997) Molecules of Emotion; Why You Feel the Way You Feel. Schribner.
Pert, C. and Marriot, N. (2006) Everything You Need to Know to Feel Go(o)d. Hay House.
Polianova, M.T. et al. (2005) Chemokine receptor-5 (CCR5) is a receptor for the HIV entry inhibitor peptide T (DAPTA). Antiviral Research67, 83-92.
Pollitica, M. et al. (2007) Profound anti-HIV-1 activity of DAPTA in monocytes/macrophages and inhibition of CRR5-mediated apoptosis in neuronal cells. Antiviral Chemistry and Chemotherapy 18, 285-295.
Quan, N. and Banks, W.A. (2007) Brain-immune communication pathways. Brain, Behaviour and Immunity 21, 727-735.
Raberg, L. et al. (2002) Basal metabolic rate and the evolution of the adaptive immune system. Proceedings of The Royal Society of London, B 269, 817-821.
Rolff, J. (2007) Why did the acquired immune system of vertebrates evolve? Developmental and Comparative Immunology 31,476-482.
Rosi, S. et al. (2005) Chemokine receptor 5 antagonist D-ala-peptide T-amide reduces microglia and astrocyte activation within the hippocampus in a neuroinflammatory rat model of Alzheimer’s disease. Neuroscience 134, 671-676.
Ruff, M.R. et al. (2003) Update on D-ala-peptide T-amide (DAPTA); a viral entry inhibitor that blocks CRR5 chemokine receptors. Current HIV Research 1, 51-67.
Salles, A. et al. (2013) Barcoding T cell calcium response diversity with methods for automated and accurate analysis of cell signals (MAAACS). PLoS Computational Biology 9, e1003245.
Shatz, C.A. (2009) MHC class I: an unexpected role in neuronal plasticity. Neuron 64, 40-45.
Smith, E.M. and Blalock, J.E. (1988) A molecular basis for interactions between the immune and neuroendocrine systems. International Journal of Neuroscience38, 455-64.
Wahl, S.M. et al. (2006) HIV accomplices and adversaries in macrophage infection. Journal of Leukocyte Biology 80, 973-983.
Weigent, D.A. et al. (1990) Bidirectional communication between the neuroendocrine and immune systems. Common hormones and hormone receptors. Annals of the New York Academy of Sciences579, 17-27.
Wrona, D. (2005) Neural–immune interactions; an integrative view of the bidirectional relationship between the brain and the immune systems. Journal of Neuroimmunology 172, 38-58.
Yong, V.W. and Rivest, S. (2009) Taking advantage of the systemic immune system to cure brain diseases. Neuron 64, 55-60.
Yuan, S. et al. (2014) Amphioxus as a model for investigating evolution of the vertebrate immune system. Developmental & Comparative Immunology (in press).
Blind evolution gives eyeless fish sleepless nights
As the bats chatter above you in the cavern roof, their droppings rain down into the pool below. The floor is silky with bacteria. It’s cold and still. A musty tang lingers in the air.
You are completely blind. You rely on hairs in your skin to feel for movement in the water.
You are hungry. Something is disturbing the mud; you can smell it! You follow the scent trail and grab at it. It feels like a shrimp.
Things touch and nip you… You are afraid of being eaten. You move slowly, dozing for short periods, but don’t sleep.
You have been here for… How long? Can you tell this without a sense of day and night?

The enhanced touch and taste senses of cave fish are also found in fish that hunt in murky waters, e.g. this channel catfish (Ictalurus punctatus). These fish have touch sensitive ‘whiskers’ (barbels) and a high den... moresity of chemoreceptors (taste organs) over their bodies, making them into a ‘tactile, swimming tongue’. These senses are more important to this fish than vision, hence their eyes are small (Image: Wikimedia Commons)
Mexico’s blind cave fish show many of the adaptations found in animals from cave ecosystems across the world. They have lost their eyes, and instead have chemoreceptors (‘taste buds’) scattered over their skin, allowing them to follow ‘pathways’ of chemical concentration (chemotaxis). Touch-sensitive hairs around the mouth, along with a well-developed lateral line system, enable them to sense tiny water currents caused by prey and other fish.
In these caves, as in the deep sea, food is always in short supply. To avoid being eaten by other fish they must remain vigilant, and so may ‘doze’ but do not sleep. These fish have a low metabolic rate to conserve energy, and build up reserves of body fat .
Why do these fish and other cave dwellers go blind? One explanation is that eye loss is neutral to their survival. When a characteristic is no longer essential to survival, mutations (mistakes in the DNA) that cause crucial genes to cease working are not selected against. They are then passed to the next generation ‘at random’.

Random bead sampling experiments show how certain forms of a gene can become quickly widespread in a few generations in a small isolated population. This is known as ‘genetic drift’ (Image: Wikimedia Commons)
These small populations of cave fish were likely founded from only a few individuals. We could anticipate that neutral mutations present in these founders would become widespread in the population by chance. However these cave fish have evolved separately multiple times. If the loss of eyes and skin pigment were a random, neutral process, we could expect some of these populations to have retained their sight and colour. Also the PAX6 gene, used to build eyes in nearly all animals, is present and working in these fish.
Another possibility is energy conservation. Eyes are costly to build and maintain, so disposing of them in this energy-poor environment seems a sensible option. This however doesn’t fit the facts. Eye cups are present in day-old embryos. By day two, the lens cells are beginning to grow and divide, but in these cave fish a process of deliberate ‘cell death’ destroys them as they arise. This strategy seems neither neutral nor particularly economic.
So what is really driving the evolution of these pale, sightless and sleepless fish? Genetic studies are providing some alternative answers that shine light into how evolution really works.
Why do cave fish kill off their own eyes?

A cross-section of a mouse eye showing the PAX6 protein, visible here in green thanks to a fluorescent tag. PAX6 is a transcription factor; a protein that controls the actions of many other genes. Its biological role is... more to define which cells develop into eyes in all vertebrate embryos. The PAX6 gene functions in the Mexican blind cave fish, but works at less intensity than in sighted fish. Cave fish eyes develop normally for the first 2 days in the embryo, then stop growing, and the cells degenerate. These fish appear eyeless because other tissues expand and cover what remains of the eye cups (Image: Wikimedia Commons)
The PAX gene plays a key role in the development of eyes in vertebrate embryos. PAX6 action is reduced by increases in the activity of another gene, called HEDGEHOG whichdefines boundaries between cell types and promotes development of the fish’s lateral line, jaws, teeth and taste buds. HEDGEHOG is more active in cave fish embryos than in their sighted sister species, causing the lateral line cell zone and the jaw to expand. As it does so, this also switches off PAX6 and halts eye development.
This trade-off between ‘touch and taste’ versus ‘sight’ enhances the very senses that the cave fish needs in its ever-dark world. Eye shrinkage, which reduces the energy budget, is a secondary effect of selection for these other senses. This subtle change in the key interaction of two developmental genes at a critical stage can radically alter a gene’s effect and the body which results.
What does enhanced touch sensitivity provide for these fish?
Try stroking your eyebrow against the direction of hair growth. Now stroke your forehead. Which feels like a stronger movement?

A school of sardines shoaling together with precise coordination.Fish have an awareness of space that is provided by their lateral line system. This sensory mode is able to pick up changes in turbulence around rocks and... more other obstacles, and to detect the movements of other fish. This is how shoals are able to swim together without colliding (Image: Wikimedia Commons)
Hairs in our skin enable us to be more sensitive to touch. The Mexican blind cave fish have a lateral line systemlike all fish. All lateral line systems use tiny hair cells to detect changes in water movement, but in these cave dwellers it is unusually sensitive.
These fish also have a behaviour which seems counter-intuitive. If introduced into a new environment, instead of slowing down and moving cautiously, they swim faster. The reason is that more rapid movements increase the flow of information to the hair cells. This expands their awareness, providing a larger ‘hydrodynamic image’ of their world. Also at greater speeds, the water layer that clings to their skin is reduced, helping to make this image ‘sharper’.
Why are cave fish so pale?

Cave animals typically lose their colour and cease to develop eyes, like this Texas Blind Salamander (Eurycea rathbuni). Cave ecosystems have limited air exchange with above ground. Often the air is low in oxygen. This ... morefilm clip shows the salamander’s external gills, a juvenile characteristic retained here in the adult form in order to capture enough oxygen gas (Image: Wikimedia Commons)
The melanin-based pigments in our skin and those of other animals provide colour and pattern, but they have a more ancient evolutionary role: to protect us from damage by ultraviolet light. Without sunlight, cave animals typically are colourless. In Mexican cave fish this is connected to a loss-of-function mutation (in the gene oca2), controlling the first step of pigment production.
Independently evolved populations of cave fish are colourless thanks to unique mutations in this same gene. This is curious. If pigment loss occurred by chance, we would expect that some populations would have shut down later stages of pigment production, as this would give the same effect. This specific mutation suggests instead that pigment loss has been actively selected.
Pigment loss may conserve energy, and it is possible that mutating the genes controlling later biosynthetic stages may have other effects that reduce fitness. However a more compelling explanation is that shutting down oca2 increases the availability of tyrosine, an amino acid. What is so special about tyrosine?

Panellus stipticus is a fungus that grows on dead wood. The bioluminescent strain is nicknamed “glow wood” (Image: Wikimedia Commons)
Neurotransmitter production depends upon the tyrosine supply. The fish produces the neurotransmitters dopamine and noradrenaline (norepinephrine), and the hormone adrenaline (epinephrine), from tyrosine. Cavefish brains have higher concentrations of these chemicals than brains of sighted fish. Noradrenaline and adrenaline provoke the ‘fight or flight response; in these fish they are linked to the minimal amounts of sleep and the ability to engage in unusually fast foraging.
Adaptation to life in caves has produced a range of animals with some remarkably similar characteristics. It seems that Mexico’s cave fish have made an evolutionary trade-off. Faster swimming and an enhanced sensitivity to touch and taste comes at the cost of eyes and skin colour. And it keeps them awake in the dark.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Bibliowicz, J, et al. (2013) Differences in chemosensory response between eyed and eyeless Astyanax mexicanus of the Rio Subterráneo cave. EvoDevo 4, e25.
Bilanžija, H. et al. (2013) A potential benefit of albinism in Astyanax cave fish: down-regulation of the oca2 gene increases tyrosine and catecholamine levels as an alternative to melanin synthesis. PLoS ONE 8, e80823.
Burt de Perera, T. and Braithwaite, V.A. (2005) Laterality in a non-visual sensory modality — the lateral line of fish. Current Biology 15, R241-R242.
Coombs, S. et al. (2000) Hydrodynamic image formation by the peripheral lateral line system of the Lake Michigan mottled sculpin, Cottus bairdi. Philosophical Transactions of the Royal Society of London, B 355, 1111-1114.
Ćurčić-Blake, B. and van Netten, S.M. (2006) Source location encoding in the fish lateral line canal. Journal of Experimental Biology 209, 1548-1559.
Horstkotte, J. et al. (2010) Predation by three species of spiders on a cavefish (Poecilia mexicana, Poeciliidae) in a Mexican sulphur cave. Bulletin of the British Arachnological Society 15, 55-58.
Jeffery, W.R. (2001) Cavefish as a model system in evolutionary developmental biology. Developmental Biology 231, 1–12.
Jeffery, W.R. (2009) Regressive evolution in Astyanax cave fish. Annual Review of Genetics 43, 25-47.
Jeffery, W.R. et al. (2003) To see or not to see: evolution of eye development in Mexican Blind Cavefish. Integrative Comparative Biology 43, 531–541.
Mueller, K.P. et al. (2014) Sunscreen for fish: the co-option of UV light protection for camouflage. PLoS ONE 9, e87372.
Niven, J.E. (2008) Evolution: convergent eye losses in fishy circumstances. Current Biology 18, R27–R29.
Rétaux, S. and Casane, D. (2013) Evolution of eye development in the darkness of caves: adaptation, drift, or both? EvoDevo 4, 26.
Sanford, W.E. et al. (2006) Research Opportunities in Interdisciplinary Ground-Water Science in the U.S. Geological Survey. Circular 1293. U.S. Geological Survey.
Strickler, A.G. et al. (2007) The lens controls cell survival in the retina: evidence from the blind cavefish Astyanax. Developmental Biology 311, 512-523.
Tian, N.M. and Price, D.J. (2005) Why cavefish are blind. BioEssays 27, 235-238.
Wilkens, H. and Strecker, U. (2003) Convergent evolution of the cavefish Astyanax (Characidae, Teleostiei): genetic evidence from reduced eye-size and pigmentation. Biological Journal of the Linnean Society 80, 545–554.
Wilkens, H. (1988) Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces). Evolutionary Biology 23, 271–367.
Yamamoto, Y. et al. (2004) Hedgehog signalling controls eye degeneration in blind cavefish. Nature 431, 844-847.
What the fish have to say about how we found a voice
A lone singer waits for darkness.
Dusk falls. As the waves push and pull at the sand with a steady rhythm, he revs up his vocal muscles for this, his love song.
He begins to hum. His baritone burr becomes louder and louder, booming across the bay. After a few minutes, another voice joins in, slightly off pitch.
They sing for over an hour. Local residents head indoors, slamming windows to block out the noise.
The male plainfin midshipman fish has evolved to sing; not for ‘fun’ but to attract females to lay their eggs in his rocky burrow. The call advertises his suitability to safeguard first the eggs and later the juvenile fry. We usually associate parental care with mammals and birds. For these territorial nesting fish, protection improves the survival of young at their most vulnerable life stage, which confers a considerable selectable advantage.

Sneaker male fish ‘cuckold’ the parental males. A cuckold is a man with an unfaithful wife, resulting in him bringing up someone else’s offspring. This term comes from the common cuckoo (Cuculus canorus), brood pa... morerasites which substitute their eggs into the nests of other birds. Their eggshell patterns match that of their smaller songbird host, so the surrogate parents (here a reed warbler, Acrocephalus scirpaceus) accept and rear the cuckoo’s outsized offspring (Image: Wikimedia Commons)
However these male fish come in two forms. These other males are smaller, look like females, and like females they don’t sing. When a real female is present they enter the nest and release sperm in the hope of fertilising some of the eggs. Extreme competition for nest sites and breeding partners is thought to have selected for the evolution of these ‘sneaker’ males. Male ‘cross-dressing’ cuckolds have been found in other animal species with extreme between-male competition for mates, some cuttlefish, lizards and dung beetles.
Singing male midshipman fish develop larger and more complex networks of vocal neurons in the brain than non-singers. These networks, together with others that control the sense of hearing, become more sensitive when the levels of sex hormones rise in the fish’ body. These chemicals peak during the spawning season, prompting the males to sing and making the females more responsive.
In some ways the fish brain is a simpler version of our own, and other tetrapods. Studying differences between the brains of these singing and non-singing male fish shows us how mate selection may have first prompted our ancestors to evolve a voice.
How does the male midshipman fish make his song?
The plainfin midshipman is one of several species of vocal fish that nest in the intertidal zone, creating a linear ‘lek’ along the coast. Singing males hum by contracting a pair of sonic muscles attached to the swim bladder. This pressurised air sac, used for buoyancy, shares developmental origins with our lungs and helps the fish amplify his own voice. Fast, synchronised contractions of the sonic muscles vibrate this ‘stiff-walled balloon’, generating sounds.
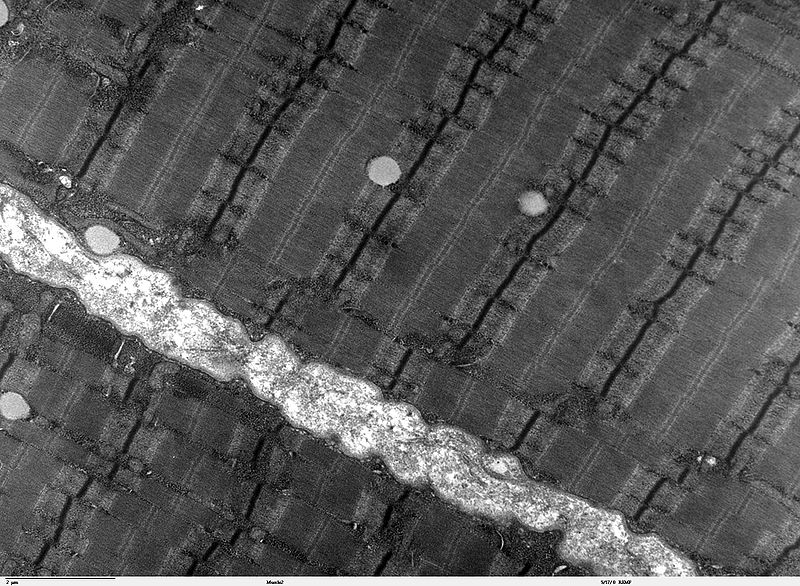
Skeletal muscles appear to have ‘stripes’ of fibres when seen under the microscope. This transmission electron microscope image shows human skeletal muscle fibres close up. The banding patterns visible here results ... morefrom overlapping strands of actin and myosin proteins. Where the actin fibres overlap, they show up as the dark lines under the electron beam, known as Z lines. In plainfin midshipman singing males, the sonic muscle actin fibres overlap more, giving these fibres their unusually high tensile strength, and making the Z lines unusually wide and pronounced (Image: Wikimedia Commons)
All midshipman fish have sonic muscles. In singing males these muscles are six times larger than in females and ‘sneaker’ males. The singer’s muscle fibres are larger, four times as numerous, and surrounded by numerous mitochondria; the cell’s ‘power generators’. Only these powerful muscles and a steady energy supply can sustain their hour-long mating call.
What inspires him to sing?
Singing males call only during the spawning season, and only at night. The hormone melatonin, produced by the pineal gland, regulates this and other daily (circadian) and seasonal rhythms in the physiology and behaviour of vertebrates. Longer hours of daylight in the spring lowers melatonin production, allowing the higher brain centres to release neurotransmitters. These small protein signals trigger the production of sex hormones, which initiate nest building and singing behaviour in midshipman parental males.

A male and female Superb Fairy wren (Malurus cyaneus) from Western Australia. These birds pair-bond to raise their brood, although females often mate covertly with other males (cuckoldry). Male fairy wrens have unusuall... morey large testes (and hence high testosterone levels) for their body size, compared with similarly sized monogamous birds. This pattern is seen in other vertebrates where females mate with several males. Producing more sperm (by having larger testes) significantly affects their chances of breeding success through better sperm competition. ‘Sneaker’ male midshipman fish also have large testes relative to their body size; their limited opportunities to fertilise a female’s eggs means that if they are to succeed, their sperm must be highly competitive (Image: Wikimedia Commons)
Both singing and ‘sneaker’ males produce the male hormone testosterone. However singers also produce a related chemical, 11-ketotestosterone, which enhances the performance of the vocal brain’s neural networks, and increases the growth of their sonic muscles.
The larger bodies of these singing males means they take longer to reach reproductive size, but potentially can mate with more females. Sneaker males have the advantage of maturing quickly but the trade-off is that their reproductive success is uncertain.
How is the fish’s brain seasonally rewired for sound?
In singing males, seasonally high levels of 11-ketotestosterone make the vocal parts of the brain more responsive, prompting them to initiate their humming calls. These brain regions contain ‘receptors’; that is protein ‘signal receivers’ that recognise the hormonal messages. As the hormone binds, the receptor changes shape into an active form and in turn modifies the genes which are employed by the vocal neurons to change their function.

A computer generated image of the human androgen receptor protein (coloured spirals) binding to a molecule of testosterone (in white). These signal decoding proteins bind testosterone, and become able to bind to short t... morearget sequences in the DNA. This affects which genes are being copied by the cell into RNA and used to build new proteins. More testosterone makes the fish’s nerve cells more sensitive to signals from other cells, triggering them to ‘fire’ more readily (Image: Wikimedia Commons)
In the part of the female fish’ ear that is the functional equivalent of our cochlea (the human hearing organ), oestrogen hormones are ‘seen’ by receptor proteins in a similar way. This renders her hearing more sensitive within the specific vocal range of the male’s droning call, enabling her to pick up its subtle nuances and high harmonics.
High oestrogen levels are also linked to better hearing in frogs and humans.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Arch, V.S. and Narins, P.M. (2009) Sexual hearing: the influence of sex hormones on acoustic communication in frogs. Hearing Research 252, 15-20.
Bass, A.H. (1990) Sounds from the intertidal zone: vocalizing fish. Bioscience 40, 249-258.
Bass, A.H. (2008) Steroid dependent plasticity of vocal motor systems: novel insights from a teleost fish. Brain Research Reviews 57, 299-308.
Bass, A.H. et al. (2008) Evolutionary origins for social vocalisation in a vertebrate hindbrain-spinal compartment. Science 321, 417-721.
Bass, A. H. (1996) Shaping brain sexuality. American Scientist 84, 352-363.
Fergus, D.J. and Bass, A.H. (2013) Localization and divergent profiles of estrogen receptors and aromatase in the vocal and auditory networks of a fish with alternative mating tactics. Journal of Comparative Neurology 521, 2850-2869.
Foran, C.M. and Bass, A.H. (1999) Preoptic GnRH and AVT: axes for sexual plasticity in teleost fish. General and Comparative Endocrinology 116, 141-152.
Knapp, R. et al. (1999) Steroid hormones and paternal care in the Plainfin Midshipman fish (Porichthys notatus). Hormones and Behavior 35, 81–89.
Moller, A.P. and Briskie, J.V. (1995) Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behavioral Ecology and Sociobiology36, 357-365.
Rubow, T.K. and Bass, A.H. (2009) Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish. Journal of Experimental Biology 212, 3252-3262.
Sloglund, C.R. (1961) Functional analysis of swimbladder muscles engaged in sound production of the toadfish. The Journal of Biophysical and Biochemical Cytology 10, 187-200.
Wilczynski, W. and Ryan, M.J. (2010) The behavioural neuroscience of anuran social signal processing. Current Opinion in Neurobiology 20, 754-763.