How touching noses sheds light on what we see
Coming up for air
Within a few hours of the naval sonar drill, reports arrived of stranded beaked whales appearing over many kilometres along the coast. These animals showed signs of decompression sickness, also known as ‘the bends’.
Post-mortems on these animals revealed gas and fat bubbles in their bones and tissues.
The deeper you dive, the more the pressure forces nitrogen and oxygen from your lungs to dissolve into your body tissues. If you then surface too quickly, these gases can come out of solution and form bubbles in your blood. These can block smaller blood capillaries, cutting off the oxygen supply to the affected tissues. Decompression sickness is a recurrent risk amongst scuba-divers who breathe compressed air, and breath-holding ‘free-divers’ who make too many consecutive dives.

We have a diving reflex like other mammals. As the water hits our face, our heart slows and muscles under the skin contract, shunting blood into the centre of our body. Water pressure increases by 1 Atmosphere for every... more 10m depth. At 2 Atmospheres, the air in our lungs is half its original volume. By 50 metres (5 Atmospheres), gaseous oxygen and nitrogen dissolves into our body tissues, and fluid floods into our lungs. The human free-diving depth record is 214 metres (Image: Wikimedia Commons)
In contrast, beaked whales routinely hunt for an hour below 1000m, using echolocation. These ‘extreme divers’ do not normally experience decompression sickness, although fossils from early in their evolutionary history show that they were not immune to these problems. X-rays of the fossilised bones of more primitive whales show regions where bubbles formed inside a capillary, damaging the bone tissue and leaving a tell-tale signature.
Whale embryos initially develop rear limb buds, like land mammals. These structures are reabsorbed back into the body later in development. The fossil record, along with DNA studies, reveal that whales’ closest living relatives are cows and hippos, which share their same four-legged (tetrapod), hoofed, land-dwelling ancestors.

The hind limbs of this Spotted Dolphin embryo (Stenella frontalis) are visible as small bumps (limb buds) near the base of the tail. (Image: Wikimedia Commons)
This raises some puzzling questions:
– Why did whales’ ancestors take to the water after 300 million years on land?
– Why didn’t they re-evolve gills?
– How can they dive for so long without getting ‘the bends’?
Why did whales’ air breathing ancestors take to the water?

These North Ronaldsay sheep are descended from an Orkney population farmed here since Neolithic times. They graze along the shoreline, feeding almost exclusively on seaweed. Their rumen stomachs have an adapted bacteria... morel population which enables them to digest marine algae (Image: Wikimedia Commons)
The land-dwelling ancestors of whales may have first waded into the sea to escape from predators on land. Shallow coastal areas offered a relatively safe haven with little competition for the new food resources available in or near the water. This initial stage would have enabled these semi-aquatic ancestors of modern whales to adapt their digestive systems to a marine food source.
Fossils from the early Eocene (52Ma) show a succession of increasingly aquatic forms. From crocodile-like and otter -like amphibious hunters, developmental changes remodelled their breathing, senses, kidney function and limbs to survive better in water. By 40Ma, these early whales had flippers, a fluked tail, and could mate, birth and suckle their young without leaving the water.
At the Eocene-Oligocene boundary (around 36Ma), movement of the continental plates opened up the deep waters of the circum-Antarctic ocean. This offered new ecological roles for the deeper-diving whales. Many new whale species appeared, including ancestors of the filter-feeding baleen whales and toothed whales that hunt in deep waters using echolocation.
Why didn’t whales re-evolve gills?

A sperm whale (Physeter macrocephalus) begins a dive; Gulf of Mexico. Adaptations for cold, deep waters include insulating blubber, lungs designed to collapse under pressure, and locomotion. The fluked tail is a super-e... morefficient ‘caudal oscillator’; both the up and down strokes generate lift, like a birds’ wing. These and other whale and seal species dive deep both to forage and to escape from killer whale (Orcinus orca) attacks (Image: Wikimedia Commons)
The ability to breathe underwater like fish seems at first like a requirement for life in the sea. However despite their lack of gills, whales and dolphins are highly effective predators in both shallow and deep water.
Modern whales’ warm bodies enable their fast reflexes for hunting. Whilst swordfish and tuna have some warm muscles, most of their tissues are at sea water temperature. Were their whole bodies warm, the heat loss from their gills would be energetically too costly.
Fish gills develop from the ‘branchial arches’; bulging structures in the early vertebrate embryo. These same tissue bulges give rise to the lower jaw, the middle ear, hyoid bone and larynx in the throat of humans and other mammals. For whales and other mammals to form gills would require that they develop new embryonic structures; this would render redundant the lungs with their vast area of vascular tissue.
Breathing air enables whales to use vocal signals to coordinate their social groups and attract mates. Like land mammals, the baleen whales make vocal calls by passing a controlled air flow through the larynx. Echolocation, the alternative means of producing sound used by dolphins and other toothed whales, also requires air. Their ‘sonic lips’ generate calls in an air-filled nasal passage. Whilst many fish make sounds, their vocal abilities are simple and limited.
How do they dive for so long without getting ‘the bends’?

This diagram shows how myoglobin forms ‘alpha-helical’ spirals around a ‘haem’ co-factor. Haem’s ring-structure holds an iron atom, carrying an electrostatic charge. This attracts and holds an oxygen molecule ... more(red spheres). As carbon dioxide builds up it dissolves to form carbonic acid. This change of pH, alters the electrostatic balance, prompting myoglobin to release its oxygen. The myoglobin protein’s high positive charge also steadies the pH when cells break down sugars without oxygen and produce lactic acid (Image: Wikimedia Commons)
All mammals store oxygen in their muscles using a protein called myoglobin. Sustained activity during long foraging dives requires a lot of oxygen. Deep divers have much higher muscle myoglobin concentrations than land mammals, giving them substantial oxygen reserves.
Modern diving mammals, and deep diving fish such as tuna, have also modified their myoglobin. As early whales began to explore the deeper waters, selection resulted in better survival from individuals whose myoglobin carried a stronger positive electrostatic charge. Like positive magnetic poles, these ‘supercharged’ molecules repel each other. This keeps them in solution, allowing them to function at high tissue concentrations where most other proteins would clump together.
A supercharged form and high concentration of myoglobin makes it possible for deep diving mammals to return to the surface slowly after a prolonged dive. This behaviour avoids decompression sickness.
However when beaked whales and other species encounter naval sonar at depth, this causes them to ‘panic’ and surface too quickly, inducing ‘the bends’.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Balasse M et al. (2006) ‘Stable isotope evidence (δ13C, δ18O) for winter feeding on seaweed by Neolithic sheep of Scotland’ Journal of Zoology 270(1); 170-176
Beatty B L & Rothschild B M (2008) ‘Decompression syndrome and the evolution of deep diving physiology in the Cetacea’ Naturwissenchaft 95;793-801
Costa D P (2007) Diving physiology of marine vertebrates’ Encyclopedia of life sciences doi:10.1002/9780470015902.a0004230
Ferguson S H et al. (2012) ‘Prey items and predation behavior of killer whales (Orcinus orca) in Nunavut, Canada based on Inuit hunter interviews’ Aquatic Biosystems 8 (3); http://www.aquaticbiosystems.org/content/8/1/3
Gatesy J et al. (2013) ‘A phylogenetic blueprint for a modern whale’ Molecular Phylogenetics and Evolution’ 66:479-506
Mirceta S et al. (2013) ‘Evolution of mammalian diving capacity traced by myoglobin net surface charge’ Science 340;1234192
Nery M F et al. (2013) ‘Accelerated evolutionary rate of the myoglobin gene in long-diving whales’ Journal of Molecular Evolution 76;380-387
Noren S R et al. (2012) ‘Changes in partial pressures of respiratory gases during submerged voluntary breath hold across odontocetes; is body mass important?’ Journal of Comparative Physiology B 182;299-309
Orpin C G et al. (1985) ‘The rumen microbiology of seaweed digestion in Orkney sheep’ Journal of Microbiology 58(6); 585-596
Rothschild B M et al (2012) ‘Adaptations for marine habitat and the effect of Jurassic and Triassic predator pressure on development of decompression syndrome in ichthyosaurs’ Naturwissenchaften 99;443-448
Steeman M E et al. (2009) ‘Radiation of extant cetaceans driven by restructuring the oceans’ systematic biology 58;573-585
Thewissen J G M et al (2007) ‘Whales originated from aquatic artiodactyls in the eocene epoch of India’ Nature 450;1190-1195
Thewissen J G M et al (2006) ‘Developmental basis for hind-limb loss in dolphins and origin of the cetacean body plan’ Proceedings of the National Academy of Sciences USA 103(22); 8414–8418
Thewissen J G M and Sunil B (2001) ‘Whale origins as a poster child for macroevolution’ Bioscience 51(12);1037-1049
Tyack P L (2006) ‘Extreme diving of beaked whales’ Journal of Experimental Biology 209;4238-4253 Naturwissenchaften 99:443-448
Uhen M D (2010) ‘The origin(s) of whales’ Annual Review of Earth and Planetary Sciences 38;189-219
Uhen M D (2007) ‘Evolution of marine mammals; back to the sea after 300 million years’ The Anatomical Record 290;514-522
Williams T M (1999) ‘The evolution of cost efficient swimming in marine mammals; limits to energetic potimization’ Philosohical Transactions of the Royal Society of London series B 354;193-201
Heated eyes give swordfish deep-sea ‘night vision’
Some 300m below the ocean surface it is always twilight, and cold… The water is barely above zero. Fast-moving squid hide here from predatory fish which stay near the surface; at this depth, their nerves would be so slowed by the cold that their eyes could no longer see for them to hunt effectively.
But there are exceptions; a stealthy predator dives into this semi darkness. Whilst the swordfish’s body temperature matches that of the water, its eyes and brain, crucially, stay toasty warm at around 23⁰C.
Why do swordfish have warm eyes?
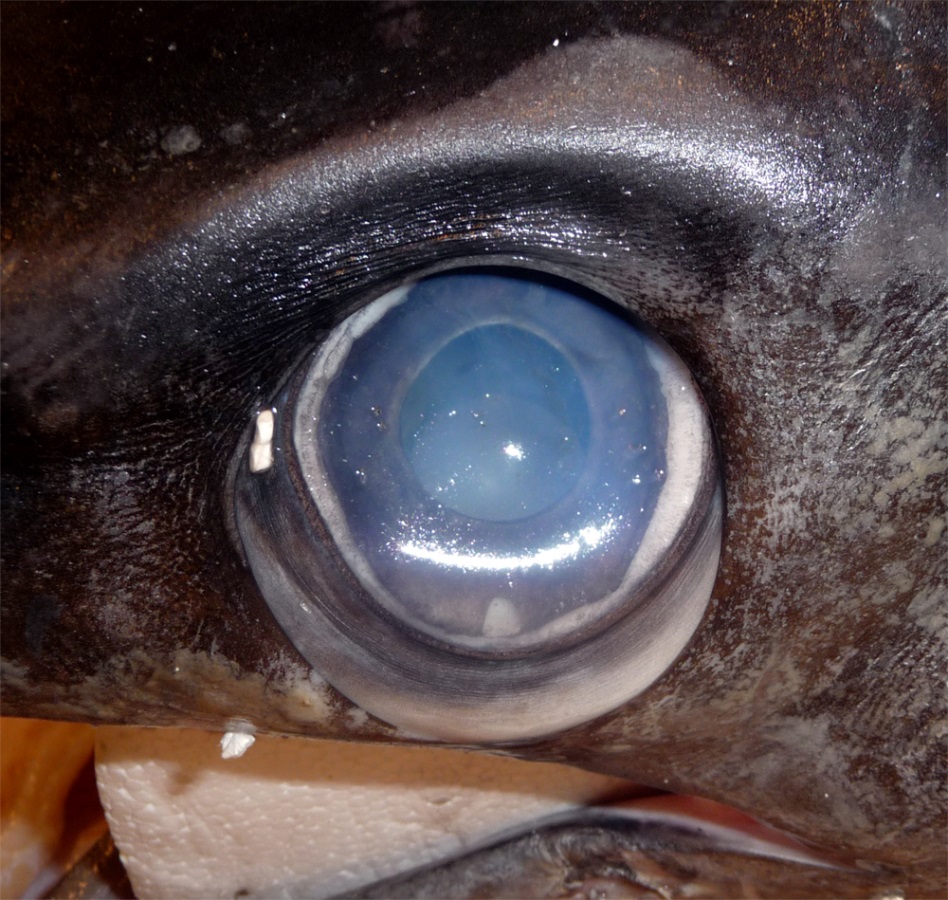
Close-up of a swordfish’s eye from a caught specimen. The eyes sit in a bony eye cup surrounded by a thick insulating layer of fatty tissue – part of which is visible here below the eyeball (Image: Wikimedia Commons... more)
A fish’s body temperature usually matches that of the water, meaning they are ‘cold blooded’ (poikilothermic). Swordfish nerves, like ours and those of other vertebrates, operate only within narrow temperature limits. The squid is also ‘cold-blooded’, but their elongated nerve cell axons however, are unusually wide, around 0.5mm diameter, and operate well in the cold, allowing them to maintain their fast movements and escape predators.
A few fish species, however, have evolved methods to generate heat in some of their tissues. Under the chilly, low light conditions of the deep sea, the warm eyes of the swordfish keep its optical nerve signals rapid. This allows it to register more visual signals per second than can other vertebrate predators. This fast image resolution ‘slows down’ apparent time and amplifies details, allowing these stealthy hunters to discern the brief flashes of silver that reveal the fleeting movements of small fish and squid.
This prompts some key evolutionary questions;
– How is the swordfish’s eye heat generated?
– How does the swordfish keep the heat localised to its eyes and brain?
– How does keeping body parts at different temperatures adapt swordfish for survival?
How is the swordfish’s eye heat generated?

Heat generation is not limited to animals. Some plants such as this Voodoo Lily (Amorphophallus titanium) have developed their own form of cellular heat generation, termed ‘non-shivering thermogenesis’. These unus... moreual plants heat parts of their floral organs to liberate scent messages into the air. This attracts insect pollinators, and may also protect its delicate reproductive tissues from the sometimes very cool night temperatures in its native tropical forest habitat (Image: Wikimedia Commons)
Swordfish eye muscles contain many brown-coloured cells that produce heat without shivering (non-shivering thermogenesis). They have a high metabolism (respiration rate) and contain many of the organelles known as mitochondria.
Mitochondria are formerly free-living bacteria found inside nearly all animal, plant and fungal cells. They ‘breathe’ for their cell, converting sugars and oxygen into carbon dioxide and water. This releases energy, which they use to pump hydrogen ions (H+, protons) from the internal matrix into their inter-membrane space. They use the chemical energy gradient this creates to produce adenosine triphosphate (ATP), life’s energy storage compound. These cellular energy factories are found in all animals and plants.
Humans and other mammals have brown adipose cells, also called ‘brown fat’. The mitochondria in these cells make very little ATP. Instead, ‘uncoupling proteins’ rearrange negatively charged fatty acids in the mitochondrial inner membranes to face into the inter-membrane space. These associate with the positively charged protons, then ‘flip-flop’, carrying them back into the matrix and dissipating the energy gradient as heat.
How does the swordfish keep the heat localised to its eyes and brain?

A pod of sperm whales (Physeter macrocephalus) diving off the coast of Mauritius. These animals are insulated by a thick layer of blubbery fat (Image: Wikimedia Commons)
When our bodies generate heat in a cold environment, this sets up an energy gradient; the bigger the differences between our internal and external temperature, the faster we cool. Warm bodies in a cold environment lose heat quickly, unless insulated. Birds use feathers, most mammals use fur and whales have blubber.
Fatty insulation over the swordfish’s skull retains heat, and helps keep its eyes and brain at a near constant temperature. These tissues are homeothermic (maintaining a stable temperature), whilst the rest of its body is poikilothermic (allowing temperatures to vary with the environment). Blood vessels supplying oxygen to the swordfish’s eye muscles are also arranged to retain heat. These vessels run in parallel, allowing outgoing veins to warm incoming arteries (this is known as a ‘counter-current’ heat exchange system).

Emperor penguins (Aptenodytes forsteri) at Atka Bay, Weddell Sea, Antarctica. The wide webbed feet of these birds have a large surface area. Reducing the skin temperature here reduces the steepness of the heat energy gr... moreadient at the place where their bodies contact the ice. This reduces the heat loss from these uninsulated body tissues (Image: Wikimedia Commons)
Insulation (fur, feathers or fat), combined with a blood supply arranged to allow counter-current heat exchange, are found in many cold-adapted animals. Lowering surface temperatures reduces the energy difference between a body and its surroundings, so minimising heat loss. Warm-bodied migrating species such as wolves and many birds from polar regions use a counter-current exchange to reduce the temperatures of their legs and feet. This means that their body parts in contact with snow or ice remain at just above zero.
How does keeping body parts at different temperatures adapt swordfish for survival?
Keeping your body at a different temperature from your environment requires a lot of energy. The swordfish’s ‘dual temperature’ body isolates the heat and keeps it in one well-insulated region; this is the most energy efficient way for these ‘wait and sprint’ hunters to survive in this environment. Tuna are another example of a fish with warm and cool tissues. Their red muscles along their spine are warm, and sustain constant ‘slow’ strokes of the tail during their long distance migrations.

An elephant dust-bathing in the ‘W du Niger’ trans-border national park, Niger Elephants cool down by ear flapping, and water and dust bathing. Their ears have a large surface area for their volume, and strong blood... more supply. Dilating the capillaries in the ears to increase blood flow to the skin allows these surfaces to lose heat to the air. At higher temperatures elephants lower their metabolic rate, reducing their resting body temperature (Image: Wikimedia Commons)
When we sweat, water evaporates and cools our skin surfaces. Dogs and many other mammals pant to evaporate water from their tongue and mouth cavity. Elephants lack both sweat glands and a panting reflex; these are possible remnants of their aquatic ancestry.
In very high temperatures they enter a whole-body heterothermic state. They slow their metabolism, lowering their morning body temperature. They then absorb daytime heat, raising their temperature above 36.7⁰C, and radiate this ‘stored’ heat at night.
Varying the temperature at times like elephants, or in certain tissues like swordfish, is known as heterothermy.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Carey, F.G. (1982) A brain heater in the swordfish. Science 216, 1327-1329.
Fritsches, K.A. et al. (2005) Warm eyes provide superior vision in swordfishes. Current Biology 15, 55-58.
Guderley, H. et al. (2005) Why are some mitochondria more powerful than others; insights from comparisons of muscle mitochondria from three terrestrial vertebrates. Comparative Biochemistry and Physiology, B 142, 172-180.
Hulbert, A.J. et al. (2006) How might you compare mitochondria from different tissues and different species? Journal of Comparative Physiology, B 176, 93-105.
Kowaltowski, A.J. (2000) Alternative mitochondrial functions in cell physiopathology; beyond ATP production. Brazilian Journal of Medical and Biological Research 33, 241-250.
Nespolo, R.F. et al. (2011) Using new tools to solve an old problem; the evolution of endothermy in vertebrates. Trends in Ecology and Evolution 26, 414-423.
Warrand, E.J. and Locket, N.A. (2004) Vision in the deep sea. Biological Reviews 79, 671-712.
Weissenbrock, N.M. et al. (2012) Taking the heat; thermoregulation in Asian elephants under different climatic conditions. Journal of Comparative Physiology, B 182, 311-319.
Making waves; how moving our arms as we talk signals our ‘inner fish’
The old Jewish Cemetery; Venice, 1790.
Goethe loosens the earth from the skull, and holds it up to the sun.
Turning the fractured bone back and forth, he gasps. A series of marks appear inside the cavity, reminding him of the vertebrae. He has looked at this pattern many times, but without seeing what lights up before him today.
Here is the shadow of a blueprint; the ‘primal repeating units’ of animal bodies, from which their many variations form.
Puzzled, Götze watches his master’s eyes shine with delight.
Watch this baby babbling; her limbs move, often in time with her sounds. the coupling of gestures and vocal calls are widespread amongst social vertebrates. And the story began with fish.

Watch this baby babbling.The rhythmical arm and leg movements of human infants as they vocalise reveals some ancient neural wiring, inherited from our common vertebrate ancestors, and now shared with other modern verteb... morerates from elephants through reptiles, amphibians and birds to fish (Image: Wikimedia Commons)
The rhythm of our breath keeps us alive. Conscious muscle movements are made through spinal nerve reflexes, but like our heart beats, the repeating sequences of muscle actions which fill and empty our lungs are outside our conscious awareness.
The movements behind repetitive activities like breathing are driven by rhythmic nerve impulses from ‘neural oscillators’. These pattern-generating circuits, located in the central nervous system, are known as ‘Central Pattern Generators’.
To breathe, to speak and to swallow we use the same internal tube; that is our throat (the pharynx). These activities are necessarily exclusive; consider what happens when a crumb ‘goes down the wrong way’. Speech therefore needs to be coordinated with our breathing.

The male club-winged manakin (Machaeropterus deliciosus) from the cloud forests of Ecuador makes sounds by rapid wing vibrations. This rhythmic movement is driven by the vertebrate vocal central pattern generators. The ... moreline drawing (shown right), from Charles Darwin’s book The descent of man, shows how the male birds’ secondary flight feathers (top ) are modified for sound (the equivalent feathers from the female bird are shown in the bottom row). Watch. (Images: Wikimedia Commons)
We make vocal sounds by passing air through the larynx as we breathe out, at the same time as vibrating our vocal folds (vocal cords). These actions involve coordinating a sequence of repetitive movements inside the throat with the repeating muscle actions that drive our breath.
Communicating with sound evolved long before animals emerged from the sea onto land and began to breathe air. Many fish use pectoral fin movements as communication gestures; some also generate sounds by fin waving.
In species of vocal fish, the calls are coordinated with these pectoral fin signals. Significantly, the muscles operating these social communication cues are controlled using the same neural oscillator ‘module’.
‘Central Pattern Generators’ are neural oscillators that generate a rhythmic output, used to control repeating muscle movements. These ‘neural metronomes’ were first discovered in insects, and produce their steady pulse without any sensory stimulus. In contrast, our other nerves operate on a ‘stimulus-response’ basis.

The predictable and repetitive movements we use for breathing, chewing and walking can speed up and slow down, but the sequence in which these muscles work (the oscillatory cycle) does not change. Oscillators are known ... morein mechanical, chemical and biological systems. This simple (undampened) oscillating spring can alter its speed, but the nature of the movement remains the same (Image: Wikimedia Commons)
Central Pattern Generators reveal what can be called our ‘deep homology’. First discovered in insects, all vertebrates, including ourselves, have these ancient neural circuits. They links vocal calls with gestures, and coordinate our ‘fins’ with our speech.
How do Central Pattern Generators work?
We consciously control our limbs through spinal nerve reflex arcs. In contrast, rhythmic movements controlling oscillating cycles are driven by Central Pattern Generators (CPGs). These autonomous modules in the central nervous system produce a rhythmic output (like a neural ‘black box’). CPG modules are comprised of a dense interconnected local network of neurons; a neural ‘node’.
These nodes are organised into three levels, each with a different function, and in each case, the parts of the circuit ‘higher’ in this organisation regulate the outputs of those below.
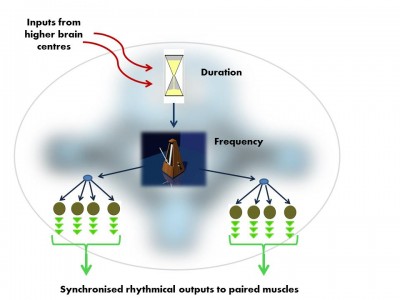
The vocal Central Pattern Generator used to produce basic signals for social communication, is organised in much the same way in fish, frogs, birds and mammals. Like all Pattern Generator modules it has a hierarchical o... morerganisation.i. pre-pacemaker cells set the duration of the output,ii. pacemaker neurons set the frequency of the regular nerve impulseiii. Motor neurons transmit the pacemaker’s rhythmic output to the muscles (Image: Wikimedia Commons)
In the developing embryo there are functional units, ‘segments’ which give rise to our vertebrae and their associated nerves and muscles. The nerves from each of these ‘segments’ form our local sensory spinal reflexes and also the CPG modules. As needed, higher brain centres trigger these ‘neural motors’ to produce their rhythmical nerve impulses and drive all of our rhythmical movements from walking to chewing.
CPGs controlling rhythmic movements of the tongue, throat and breathing (including the vocal neural oscillator module) are in the lower brainstem and neck. The CPGs that drive the rhythm of our walking are low down in the spinal cord, in the thoracic and lumbar regions.
Why don’t we sound like fish?

Vocal fish such as this Oyster toadfish (Opsanus tau) produce calls in one of two ‘output modes’. This is controlled by testosterone, which reduces the threshold of nerve stimulus needed to initiate calls. In ‘nor... moremal’ mode, these fish are able to sustain only slow rhythmic grunts. ‘Mating mode’ speeds up these sounds into a buzzing drone. Mating calls are made only at night during the spawning season, when testosterone levels are high.In this video clip the closely related plainfin midshipman fish (Porichthys notatus) demonstrates both call types (Image: Wikimedia Commons)
Toadfish vocalise with either a sequence of repetitive grunts during aggressive encounters or the prolonged drone of their mating call. In both cases, each nerve impulse from the vocal pattern generator produces a single synchronised contraction in their sonic muscles; this muscle pair flexes the rigid walls of the swimbladder, producing a ‘grunt’. This sound receives no further processing. As a result, its tone is rather mechanical.
Our voice, like that of frogs, birds and mammals, also begins with this simple rhythmic sound pulse. This initial sound is then processed into croaks, calls songs and speech. Our neck allows us to create resonant areas in the throat which amplify certain frequencies. Pitch is affected by vocal fold (vocal cord) tension, and manipulation of our tongue and lips produces precisely articulated words.
Why is ‘talking with our hands’ still a part of our language?

This Siamese fighting fish (Betta splendens) uses rapid pectoral fin movements as a posturing signal during competitive displays with other males. Watch displaying fish in adjacent tanks using pectoral fins signals (Image: Wikimedia... more Commons)
Many vocal fish make synchronised gestures with their front (pectoral) fins during mating calls. These motor nerve connections from our ancient common ancestor are retained in other vertebrates.
People blind from birth move their hands when they talk. Our vocal Pattern Generator circuits connects with both our larynx and pectoral muscles, coordinating our speech with our ‘body language’. We subconsciously move our hands as we communicate thanks to these rhythmic central circuits. As in all other vertebrates, we have inherited these from (as Palaeontologist Neil Shubin puts it) our ancestral ‘inner fish’.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Aboitiz, F. (2012) Gestures, vocalizations, and memory in language origins. Frontiers in Evolutionary Neuroscience 4, e2.
Bass, A.H. and Chagnaud, B.P. (2012) Shared developmental and evolutionary origins for neural basis of vocal-acoustic and pectoral-gestural signalling. Proceedings of the National Academy of Sciences, USA 109 (Suppl.1), 10677-10684.
Bass, A.H. et al. (2008) Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science 321, 417-421.
Bostwick, K.S. (2000) Display behaviors, mechanical sounds, and evolutionary relationships of the Club-winged Manakin (Machaeropterus deliciosus). Auk 117, 465-478.
Bostwick, K.S. et al. (2010) Resonating feathers produce courtship song. Proceedings of the Royal Society, B 277, 835-841.
Chagnaud, B.P. et al. (2012) Innovations in motoneuron synchrony drive rapid temporal modulations in vertebrate acoustic signalling. Journal of Neurophysiology 107, 3528-3542.
Dick, A.S. et al. (2012) Gesture in the developing brain. Developmental Science 15, 165–180.
Ghazanfar, A.A. (2013) Multisensory vocal communication in primates and the evolution of rhythmic speech. Behavioral Ecology and Sociobiology 67, 1441-1448.
Goethe, J.W. (1820). Zur Naturwissenschaften überhaupt, besonders zur Morphologie; cited (p. 7) in G.R. de Beer The Development of the Vertebrate Skull. Clarendon (1937).
Guthrie, S. (1996) Patterning the hindbrain. Current Opinion in Neurobiology 6,41-48.
Hanneman, E. et al. (1988) Segmental pattern of development of the hindbrain and spinal cord of the zebrafish embryo. Development 103, 49-58.
Iverson, J.M. and Thelen, E. (1999) Hand, mouth and brain: The dynamic emergence of speech and gesture. Journal of Consciousness Studies 6, 19-40.
Kelley, D.B. and Bass, A.H. (2010) Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Current Opinion in Neurobiology 20,748-53.
Marder, E. and Bucher, D. (2001) Central pattern generators and the control of rhythmic movements. Current Biology 11, R986-R996.
Shubin, N (2008) Your inner fish; a journey into the 3.5 Billion year history of the human body. Penguin Books Ltd.
Shubin, N. et al. (2009) Deep homology and the origins of evolutionary novelty. Nature 457, 818-823.
Blind evolution gives eyeless fish sleepless nights
As the bats chatter above you in the cavern roof, their droppings rain down into the pool below. The floor is silky with bacteria. It’s cold and still. A musty tang lingers in the air.
You are completely blind. You rely on hairs in your skin to feel for movement in the water.
You are hungry. Something is disturbing the mud; you can smell it! You follow the scent trail and grab at it. It feels like a shrimp.
Things touch and nip you… You are afraid of being eaten. You move slowly, dozing for short periods, but don’t sleep.
You have been here for… How long? Can you tell this without a sense of day and night?

The enhanced touch and taste senses of cave fish are also found in fish that hunt in murky waters, e.g. this channel catfish (Ictalurus punctatus). These fish have touch sensitive ‘whiskers’ (barbels) and a high den... moresity of chemoreceptors (taste organs) over their bodies, making them into a ‘tactile, swimming tongue’. These senses are more important to this fish than vision, hence their eyes are small (Image: Wikimedia Commons)
Mexico’s blind cave fish show many of the adaptations found in animals from cave ecosystems across the world. They have lost their eyes, and instead have chemoreceptors (‘taste buds’) scattered over their skin, allowing them to follow ‘pathways’ of chemical concentration (chemotaxis). Touch-sensitive hairs around the mouth, along with a well-developed lateral line system, enable them to sense tiny water currents caused by prey and other fish.
In these caves, as in the deep sea, food is always in short supply. To avoid being eaten by other fish they must remain vigilant, and so may ‘doze’ but do not sleep. These fish have a low metabolic rate to conserve energy, and build up reserves of body fat .
Why do these fish and other cave dwellers go blind? One explanation is that eye loss is neutral to their survival. When a characteristic is no longer essential to survival, mutations (mistakes in the DNA) that cause crucial genes to cease working are not selected against. They are then passed to the next generation ‘at random’.

Random bead sampling experiments show how certain forms of a gene can become quickly widespread in a few generations in a small isolated population. This is known as ‘genetic drift’ (Image: Wikimedia Commons)
These small populations of cave fish were likely founded from only a few individuals. We could anticipate that neutral mutations present in these founders would become widespread in the population by chance. However these cave fish have evolved separately multiple times. If the loss of eyes and skin pigment were a random, neutral process, we could expect some of these populations to have retained their sight and colour. Also the PAX6 gene, used to build eyes in nearly all animals, is present and working in these fish.
Another possibility is energy conservation. Eyes are costly to build and maintain, so disposing of them in this energy-poor environment seems a sensible option. This however doesn’t fit the facts. Eye cups are present in day-old embryos. By day two, the lens cells are beginning to grow and divide, but in these cave fish a process of deliberate ‘cell death’ destroys them as they arise. This strategy seems neither neutral nor particularly economic.
So what is really driving the evolution of these pale, sightless and sleepless fish? Genetic studies are providing some alternative answers that shine light into how evolution really works.
Why do cave fish kill off their own eyes?

A cross-section of a mouse eye showing the PAX6 protein, visible here in green thanks to a fluorescent tag. PAX6 is a transcription factor; a protein that controls the actions of many other genes. Its biological role is... more to define which cells develop into eyes in all vertebrate embryos. The PAX6 gene functions in the Mexican blind cave fish, but works at less intensity than in sighted fish. Cave fish eyes develop normally for the first 2 days in the embryo, then stop growing, and the cells degenerate. These fish appear eyeless because other tissues expand and cover what remains of the eye cups (Image: Wikimedia Commons)
The PAX gene plays a key role in the development of eyes in vertebrate embryos. PAX6 action is reduced by increases in the activity of another gene, called HEDGEHOG whichdefines boundaries between cell types and promotes development of the fish’s lateral line, jaws, teeth and taste buds. HEDGEHOG is more active in cave fish embryos than in their sighted sister species, causing the lateral line cell zone and the jaw to expand. As it does so, this also switches off PAX6 and halts eye development.
This trade-off between ‘touch and taste’ versus ‘sight’ enhances the very senses that the cave fish needs in its ever-dark world. Eye shrinkage, which reduces the energy budget, is a secondary effect of selection for these other senses. This subtle change in the key interaction of two developmental genes at a critical stage can radically alter a gene’s effect and the body which results.
What does enhanced touch sensitivity provide for these fish?
Try stroking your eyebrow against the direction of hair growth. Now stroke your forehead. Which feels like a stronger movement?

A school of sardines shoaling together with precise coordination.Fish have an awareness of space that is provided by their lateral line system. This sensory mode is able to pick up changes in turbulence around rocks and... more other obstacles, and to detect the movements of other fish. This is how shoals are able to swim together without colliding (Image: Wikimedia Commons)
Hairs in our skin enable us to be more sensitive to touch. The Mexican blind cave fish have a lateral line systemlike all fish. All lateral line systems use tiny hair cells to detect changes in water movement, but in these cave dwellers it is unusually sensitive.
These fish also have a behaviour which seems counter-intuitive. If introduced into a new environment, instead of slowing down and moving cautiously, they swim faster. The reason is that more rapid movements increase the flow of information to the hair cells. This expands their awareness, providing a larger ‘hydrodynamic image’ of their world. Also at greater speeds, the water layer that clings to their skin is reduced, helping to make this image ‘sharper’.
Why are cave fish so pale?

Cave animals typically lose their colour and cease to develop eyes, like this Texas Blind Salamander (Eurycea rathbuni). Cave ecosystems have limited air exchange with above ground. Often the air is low in oxygen. This ... morefilm clip shows the salamander’s external gills, a juvenile characteristic retained here in the adult form in order to capture enough oxygen gas (Image: Wikimedia Commons)
The melanin-based pigments in our skin and those of other animals provide colour and pattern, but they have a more ancient evolutionary role: to protect us from damage by ultraviolet light. Without sunlight, cave animals typically are colourless. In Mexican cave fish this is connected to a loss-of-function mutation (in the gene oca2), controlling the first step of pigment production.
Independently evolved populations of cave fish are colourless thanks to unique mutations in this same gene. This is curious. If pigment loss occurred by chance, we would expect that some populations would have shut down later stages of pigment production, as this would give the same effect. This specific mutation suggests instead that pigment loss has been actively selected.
Pigment loss may conserve energy, and it is possible that mutating the genes controlling later biosynthetic stages may have other effects that reduce fitness. However a more compelling explanation is that shutting down oca2 increases the availability of tyrosine, an amino acid. What is so special about tyrosine?

Panellus stipticus is a fungus that grows on dead wood. The bioluminescent strain is nicknamed “glow wood” (Image: Wikimedia Commons)
Neurotransmitter production depends upon the tyrosine supply. The fish produces the neurotransmitters dopamine and noradrenaline (norepinephrine), and the hormone adrenaline (epinephrine), from tyrosine. Cavefish brains have higher concentrations of these chemicals than brains of sighted fish. Noradrenaline and adrenaline provoke the ‘fight or flight response; in these fish they are linked to the minimal amounts of sleep and the ability to engage in unusually fast foraging.
Adaptation to life in caves has produced a range of animals with some remarkably similar characteristics. It seems that Mexico’s cave fish have made an evolutionary trade-off. Faster swimming and an enhanced sensitivity to touch and taste comes at the cost of eyes and skin colour. And it keeps them awake in the dark.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Bibliowicz, J, et al. (2013) Differences in chemosensory response between eyed and eyeless Astyanax mexicanus of the Rio Subterráneo cave. EvoDevo 4, e25.
Bilanžija, H. et al. (2013) A potential benefit of albinism in Astyanax cave fish: down-regulation of the oca2 gene increases tyrosine and catecholamine levels as an alternative to melanin synthesis. PLoS ONE 8, e80823.
Burt de Perera, T. and Braithwaite, V.A. (2005) Laterality in a non-visual sensory modality — the lateral line of fish. Current Biology 15, R241-R242.
Coombs, S. et al. (2000) Hydrodynamic image formation by the peripheral lateral line system of the Lake Michigan mottled sculpin, Cottus bairdi. Philosophical Transactions of the Royal Society of London, B 355, 1111-1114.
Ćurčić-Blake, B. and van Netten, S.M. (2006) Source location encoding in the fish lateral line canal. Journal of Experimental Biology 209, 1548-1559.
Horstkotte, J. et al. (2010) Predation by three species of spiders on a cavefish (Poecilia mexicana, Poeciliidae) in a Mexican sulphur cave. Bulletin of the British Arachnological Society 15, 55-58.
Jeffery, W.R. (2001) Cavefish as a model system in evolutionary developmental biology. Developmental Biology 231, 1–12.
Jeffery, W.R. (2009) Regressive evolution in Astyanax cave fish. Annual Review of Genetics 43, 25-47.
Jeffery, W.R. et al. (2003) To see or not to see: evolution of eye development in Mexican Blind Cavefish. Integrative Comparative Biology 43, 531–541.
Mueller, K.P. et al. (2014) Sunscreen for fish: the co-option of UV light protection for camouflage. PLoS ONE 9, e87372.
Niven, J.E. (2008) Evolution: convergent eye losses in fishy circumstances. Current Biology 18, R27–R29.
Rétaux, S. and Casane, D. (2013) Evolution of eye development in the darkness of caves: adaptation, drift, or both? EvoDevo 4, 26.
Sanford, W.E. et al. (2006) Research Opportunities in Interdisciplinary Ground-Water Science in the U.S. Geological Survey. Circular 1293. U.S. Geological Survey.
Strickler, A.G. et al. (2007) The lens controls cell survival in the retina: evidence from the blind cavefish Astyanax. Developmental Biology 311, 512-523.
Tian, N.M. and Price, D.J. (2005) Why cavefish are blind. BioEssays 27, 235-238.
Wilkens, H. and Strecker, U. (2003) Convergent evolution of the cavefish Astyanax (Characidae, Teleostiei): genetic evidence from reduced eye-size and pigmentation. Biological Journal of the Linnean Society 80, 545–554.
Wilkens, H. (1988) Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces). Evolutionary Biology 23, 271–367.
Yamamoto, Y. et al. (2004) Hedgehog signalling controls eye degeneration in blind cavefish. Nature 431, 844-847.
What the fish have to say about how we found a voice
A lone singer waits for darkness.
Dusk falls. As the waves push and pull at the sand with a steady rhythm, he revs up his vocal muscles for this, his love song.
He begins to hum. His baritone burr becomes louder and louder, booming across the bay. After a few minutes, another voice joins in, slightly off pitch.
They sing for over an hour. Local residents head indoors, slamming windows to block out the noise.
The male plainfin midshipman fish has evolved to sing; not for ‘fun’ but to attract females to lay their eggs in his rocky burrow. The call advertises his suitability to safeguard first the eggs and later the juvenile fry. We usually associate parental care with mammals and birds. For these territorial nesting fish, protection improves the survival of young at their most vulnerable life stage, which confers a considerable selectable advantage.

Sneaker male fish ‘cuckold’ the parental males. A cuckold is a man with an unfaithful wife, resulting in him bringing up someone else’s offspring. This term comes from the common cuckoo (Cuculus canorus), brood pa... morerasites which substitute their eggs into the nests of other birds. Their eggshell patterns match that of their smaller songbird host, so the surrogate parents (here a reed warbler, Acrocephalus scirpaceus) accept and rear the cuckoo’s outsized offspring (Image: Wikimedia Commons)
However these male fish come in two forms. These other males are smaller, look like females, and like females they don’t sing. When a real female is present they enter the nest and release sperm in the hope of fertilising some of the eggs. Extreme competition for nest sites and breeding partners is thought to have selected for the evolution of these ‘sneaker’ males. Male ‘cross-dressing’ cuckolds have been found in other animal species with extreme between-male competition for mates, some cuttlefish, lizards and dung beetles.
Singing male midshipman fish develop larger and more complex networks of vocal neurons in the brain than non-singers. These networks, together with others that control the sense of hearing, become more sensitive when the levels of sex hormones rise in the fish’ body. These chemicals peak during the spawning season, prompting the males to sing and making the females more responsive.
In some ways the fish brain is a simpler version of our own, and other tetrapods. Studying differences between the brains of these singing and non-singing male fish shows us how mate selection may have first prompted our ancestors to evolve a voice.
How does the male midshipman fish make his song?
The plainfin midshipman is one of several species of vocal fish that nest in the intertidal zone, creating a linear ‘lek’ along the coast. Singing males hum by contracting a pair of sonic muscles attached to the swim bladder. This pressurised air sac, used for buoyancy, shares developmental origins with our lungs and helps the fish amplify his own voice. Fast, synchronised contractions of the sonic muscles vibrate this ‘stiff-walled balloon’, generating sounds.
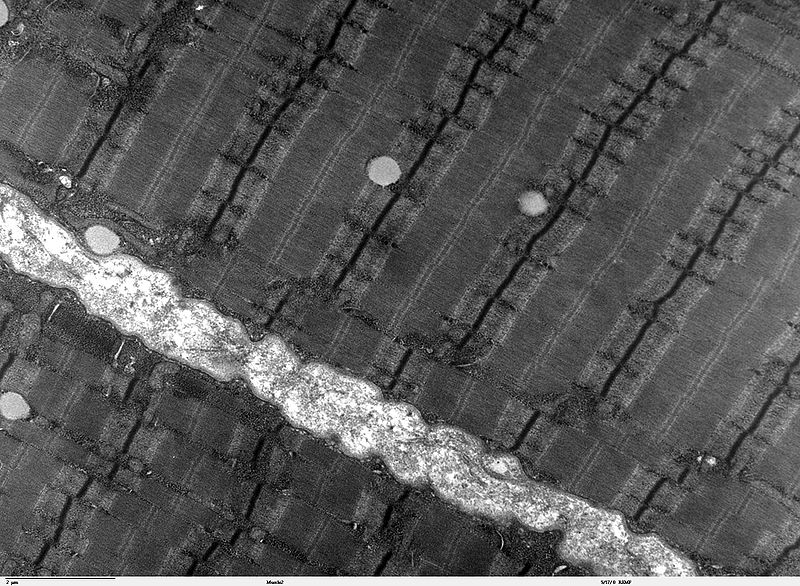
Skeletal muscles appear to have ‘stripes’ of fibres when seen under the microscope. This transmission electron microscope image shows human skeletal muscle fibres close up. The banding patterns visible here results ... morefrom overlapping strands of actin and myosin proteins. Where the actin fibres overlap, they show up as the dark lines under the electron beam, known as Z lines. In plainfin midshipman singing males, the sonic muscle actin fibres overlap more, giving these fibres their unusually high tensile strength, and making the Z lines unusually wide and pronounced (Image: Wikimedia Commons)
All midshipman fish have sonic muscles. In singing males these muscles are six times larger than in females and ‘sneaker’ males. The singer’s muscle fibres are larger, four times as numerous, and surrounded by numerous mitochondria; the cell’s ‘power generators’. Only these powerful muscles and a steady energy supply can sustain their hour-long mating call.
What inspires him to sing?
Singing males call only during the spawning season, and only at night. The hormone melatonin, produced by the pineal gland, regulates this and other daily (circadian) and seasonal rhythms in the physiology and behaviour of vertebrates. Longer hours of daylight in the spring lowers melatonin production, allowing the higher brain centres to release neurotransmitters. These small protein signals trigger the production of sex hormones, which initiate nest building and singing behaviour in midshipman parental males.

A male and female Superb Fairy wren (Malurus cyaneus) from Western Australia. These birds pair-bond to raise their brood, although females often mate covertly with other males (cuckoldry). Male fairy wrens have unusuall... morey large testes (and hence high testosterone levels) for their body size, compared with similarly sized monogamous birds. This pattern is seen in other vertebrates where females mate with several males. Producing more sperm (by having larger testes) significantly affects their chances of breeding success through better sperm competition. ‘Sneaker’ male midshipman fish also have large testes relative to their body size; their limited opportunities to fertilise a female’s eggs means that if they are to succeed, their sperm must be highly competitive (Image: Wikimedia Commons)
Both singing and ‘sneaker’ males produce the male hormone testosterone. However singers also produce a related chemical, 11-ketotestosterone, which enhances the performance of the vocal brain’s neural networks, and increases the growth of their sonic muscles.
The larger bodies of these singing males means they take longer to reach reproductive size, but potentially can mate with more females. Sneaker males have the advantage of maturing quickly but the trade-off is that their reproductive success is uncertain.
How is the fish’s brain seasonally rewired for sound?
In singing males, seasonally high levels of 11-ketotestosterone make the vocal parts of the brain more responsive, prompting them to initiate their humming calls. These brain regions contain ‘receptors’; that is protein ‘signal receivers’ that recognise the hormonal messages. As the hormone binds, the receptor changes shape into an active form and in turn modifies the genes which are employed by the vocal neurons to change their function.

A computer generated image of the human androgen receptor protein (coloured spirals) binding to a molecule of testosterone (in white). These signal decoding proteins bind testosterone, and become able to bind to short t... morearget sequences in the DNA. This affects which genes are being copied by the cell into RNA and used to build new proteins. More testosterone makes the fish’s nerve cells more sensitive to signals from other cells, triggering them to ‘fire’ more readily (Image: Wikimedia Commons)
In the part of the female fish’ ear that is the functional equivalent of our cochlea (the human hearing organ), oestrogen hormones are ‘seen’ by receptor proteins in a similar way. This renders her hearing more sensitive within the specific vocal range of the male’s droning call, enabling her to pick up its subtle nuances and high harmonics.
High oestrogen levels are also linked to better hearing in frogs and humans.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Arch, V.S. and Narins, P.M. (2009) Sexual hearing: the influence of sex hormones on acoustic communication in frogs. Hearing Research 252, 15-20.
Bass, A.H. (1990) Sounds from the intertidal zone: vocalizing fish. Bioscience 40, 249-258.
Bass, A.H. (2008) Steroid dependent plasticity of vocal motor systems: novel insights from a teleost fish. Brain Research Reviews 57, 299-308.
Bass, A.H. et al. (2008) Evolutionary origins for social vocalisation in a vertebrate hindbrain-spinal compartment. Science 321, 417-721.
Bass, A. H. (1996) Shaping brain sexuality. American Scientist 84, 352-363.
Fergus, D.J. and Bass, A.H. (2013) Localization and divergent profiles of estrogen receptors and aromatase in the vocal and auditory networks of a fish with alternative mating tactics. Journal of Comparative Neurology 521, 2850-2869.
Foran, C.M. and Bass, A.H. (1999) Preoptic GnRH and AVT: axes for sexual plasticity in teleost fish. General and Comparative Endocrinology 116, 141-152.
Knapp, R. et al. (1999) Steroid hormones and paternal care in the Plainfin Midshipman fish (Porichthys notatus). Hormones and Behavior 35, 81–89.
Moller, A.P. and Briskie, J.V. (1995) Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behavioral Ecology and Sociobiology36, 357-365.
Rubow, T.K. and Bass, A.H. (2009) Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish. Journal of Experimental Biology 212, 3252-3262.
Sloglund, C.R. (1961) Functional analysis of swimbladder muscles engaged in sound production of the toadfish. The Journal of Biophysical and Biochemical Cytology 10, 187-200.
Wilczynski, W. and Ryan, M.J. (2010) The behavioural neuroscience of anuran social signal processing. Current Opinion in Neurobiology 20, 754-763.
The reptile that almost became a fish
‘It’s head is as long as I am tall!’
‘What is it?’
‘Hmm… A giant fish? A lizard? Don’t know.’
‘Well I know! It’s a sea dragon!’
It is spring, 1811, the morning after a storm. Mary is 12 years old, and fearless. She edges across the cliff to where her brother is already working to free the fossil bones. Fragments of weathered mudstone clatter down onto the beach. The eye sockets of the huge skull are wider than the span of her hand.

A 185 million year old fossil of Ichthyosaurus acutirostris beside ammonites (Harpoceras falcifer). This specimen shows the distinctive downward (hypoocercal) bend of the spine into the lower tail fluke, characteristic ... moreof this reptile group. The outlines of the fluked tail and dorsal fin are visible; these were supported by cartilage rather than bone as in modern fish. The huge eye sockets (relative to its body size) enabled these animals to hunt by sight for shellfish, small fish and squid in dimly lit or murky waters (Image: Wikimedia Commons)
During the 200 million years that dinosaurs roamed the land, the oceans were ruled by formerly land-dwelling reptiles. Of these, ichthyosaurs adopted dolphin-like forms, plesiosaurs became sea lion-like, and mosasaurs occupied crocodile-like ‘ambush’ predator roles.
Of these, ichthyosaurs were arguably the most successful. Their multiple adaptations to a fully aquatic life included huge eyes, a stiffened, fluked tail, and the ability to mate and give birth to live young in water. Much as killer whales do today, these predators structured the marine ecosystem. Many came to resemble the modern whales, dolphins and tuna fish that now fulfil similar ecological roles. The convergence of body forms between some ichthyosaur species and tuna are particularly astonishing because these reptiles were air breathers.
Why did icthyosaurs evolve to look like modern marine animals?

Ichthyosaurs first colonised the sea 250Ma ago. The earliest known aquatic ichthyosaur, the otter-like Utatsusaurus hataii (top) fed on fish and shellfish in shallow water. After the Cretaceous-Tertiary mass extinction ... more(65Ma), the land-based ancestors of modern whales also took to water. The early whale Kutchicetus minimus (bottom) had an otter-like ecological role, and converged to evolve a similar body form. Both had an undulating swimming style which was in the horizontal plane (like an eel) for Utatsusarus, and vertical for Kutchicetus. This reflects the style of locomotion inherited from their respective ancestors (Images: Wikimedia Commons)
Life in water poses a specific set of challenges. These marine reptiles fulfilled similar ecological roles to whales, and in time evolved body forms similar to these modern mammals. This is known as convergence.
Like whales and tuna, ichthyosaurs were adapted for long distance energy-efficient swimming. The respective horizontal and vertical strokes of both ichthyosaur and dolphin tails give an equally powerful ‘lift’ in both directions, propelling these animals forward in a near straight line.
A further example of this convergence is seen in the modern otter. Unlike the Ichthyosaurs, both whales and otters had land-based mammalian ancestors with a vertically moving spine, giving them their bounding gait. The whale-like ichthyosaurs moved their tail flukes horizontally, like a modern lizard.
Like whales and tuna, ichthyosaurs were adapted for long distance energy-efficient swimming. The respective horizontal and vertical strokes of both ichthyosaur and , propelling these animals forward in a near straight line.
What can modern animals tell us about ichthyosaurs?

The ichthyosaur Stenopterygius quadriscissus (above), became widespread, in the late Jurassic and early Cretaceous (160-100Ma). Its body shape is similar to that of the Bluefin tuna (Thunnus thynnus (below). Tuna hunt f... moreish and squid at around 500m depth. This is possible because they have a high oxygen intake, fast metabolic rate, warm muscles, an energy efficient swimming style, and a higher heart rate and blood pressure than other fish (Images: Wikimedia Commons)
Modern tuna and lamnid sharks have converged into a similar ‘deep water sprint-predator’ ecological role. These long distance migrants move constantly at a moderate speed, except for short fast bursts when chasing prey. Chunky vertebrae stiffen their bodies at their core, reducing sideways movements except at the narrow ‘hinge’ before the tail. The continuously active red ‘cruising’ muscles either side of the spine are warm, in marked contrast to most other fish. In combination with large tendons, these muscles work like pulleys, flicking the fluked tail from side to side. The surrounding white muscles give extra power during short ‘sprints’.
The ichthyosaur Stenopterygius was tuna-shaped with chunky stacked vertebrae. This stiffened body form suggests that these reptiles converged on the cruise-and-sprint deep water hunting role of modern tuna and lamnids.
Did ichthyosaurs have warm muscles like whales and tuna?

Cast of a skeleton of Hawkins’ plesiosaur (Thalassiodracon hawkinsi) from the Lower Lias strata at Street in Somerset; part of England’s Jurassic coast. These rocks are rich in marine fossils of all kinds including ... morefish, ammonites and belemnites (Image: Wikimedia Commons)
The isotopic proportion between the heavy 18O and the light 16O oxygen (the ratio is given as d18O) in the bones of living fish and marine animals decreases as body temperature increases. In principle we can use cold-blooded fish fossils as a ‘thermometer’ to indicate the water temperature, and compare this against isotope-predicted body temperatures for other fossil animals from the same rocks.
Oxygen isotope data allows us to infer that Jurassic plesiosaurs and ichthyosaurs had body temperatures of around 35⁰C, much higher than that of their environment. This indicates that they could generate heat, and may well regulated their body temperatures independently of their environment (homeothermy). Modern warm bodied marine animals have to conserve their body heat. This means it is reasonable to infer that ichthyosaurs used similar methods such as a counter-current blood circulation system and/or heat-insulating blubber.
What happened to the ichthyosaurs?

Plotosaurus bennisoni; a mosasaur from the Upper Cretaceous of North America. Most mososaurs lived in shallow coastal waters, although after the disappearance of the ichthyosaurs, some evolved into similar deep water sp... morerint predators. Plotosaurus had crescent-shaped tail flukes, equipping this animal to whale-like fast pursuit behaviour (Image: Wikimedia Commons)
Ichthyosaurs dominated the world’s oceans for around 150 million years, but then disappeared from the fossil record after the mid-Cretaceous (around 95Ma). The cause of their sudden extinction remains a mystery. The empty ecological roles that this created were later filled by mosasaurs; relatives of modern monitor lizards including the Komodo dragon. In turn these reptiles died out during the Cretaceous-Tertiary mass extinction (65Ma), making way for the later evolution of modern whales, dolphins and tuna.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Benson, R.B.J. and Butler, R.J. (2011) Uncovering the diversification history of marine tetrapods: ecology influences the effect of geological sampling biases In Comparing the Geological and Fossil Records: Implications for Biodiversity Studies ( A.J. McGowan and A.B.Smith, eds). Geological Society of London Special Publications 358, 191-208.
Bernal, D. et al. (2005) Mammal-like muscles power swimming in a cold-water shark. Nature 437: 1349-1352.
Brill, R.W. (1996) Selective advantages conferred by the high performance physiology of tunas, billfishes and dolphin fish. Comparative Biochemistry and Physiology, A 113, 3-15.
Brill, R.W. et al.(2005) Bigeye tuna (Thunnus obesus) behavior and physiology and their relevance to stock assessments and fishery biology. Collective Volume of Scientific Papers ICCAT 57, 142-161. Online;http://www.iccat.es/en/pubs_CVSP.htm
Dickson, K.A. and Graham, J.B. (2004) Evolution and consequences of endothermy in fishes. Physiological and Biochemical Zoology 77, 998-1018.
Donley, J.M. (2004) Convergent evolution in mechanical design of lamnid sharks and tuna. Nature 429, 61-65.
Fröbisch, N.B. et al. (2013) Macropredatory ichthyosaur from the middle Triassic and the origin of modern trophic networks. Proceedings of the National Academy of Sciences, USA 110, 1393-1397.
Graham, J.B. and Dickson, K.A. (2004) Tuna comparative physiology. Journal of Experimental Biology 297, 4015-4024.
Houssaye, A. (2013) Bone histology of aquatic reptiles: what does it tell us about secondary adaptation to an aquatic life? Biological Journal of the Linnean Society 108, 3-21.
Korsmeyer, K.E. et al. (1996) The aerobic capacity of tunas; adaptation for multiple metabolic demands. Comparative Biochemistry and Physiology, A 113, 17-24.
Lindgren, J. et al. (2007) A fishy mosasaur: the axial skeleton of Plotosaurus (Reptilia, Squamata) reassessed. Lethaia 40,153–160.
Lindgren, J. et al. (2011) Landlubbers to leviathans: evolution of swimming in mosasaurine mosasaurs. Paleobiology 37, 445-469.
Lingham-Soliar, T. and Plodowski, G. (2007) Taphonomic evidence for high-speed adapted fins in thunniform ichthyosaurs. Naturwissenschaften 94, 65-70.
Malte, H. et al. (2007) Differential heating and cooling rates in bigeye tuna (Thunnus obesus Lowe); a model of non-steady heat exchange. Journal of Experimental Biology 210, 2618-2626.
Motani, R. et al. (1998) Ichthyosaurian relationships illuminated by new primitive skeletons from Japan. Nature 393, 255-257.
Motani, R. (2002) Scaling effects in caudal fin propulsion and the speed of ichthyosaurs. Nature 415, 309-312.
Motani, R. (2002) Swimming speed estimation of extinct marine reptiles: energetic approach revisited. Palaeobiology 28, 251-262.
Motani, R. (2010) Warm-blooded “Sea Dragons”? Science 328, 1361-1362.
Rothschild, B. M. et al. (2012) Adaptations for marine habitat and the effect of Triassic and Jurassic predator pressure on development of decompression syndrome in ichthyosaurs. Naturwissenschaften 99, 443-448.
Syme, D.A. and Shadwick, R.E. (2011) Red muscle function in stiff bodied swimmers: there and almost back again. Philosophical Transactions of the Royal Society, B 366, 1507-1515.
Thewissen, J.G.M. and Bajpai, S. (2009) New skeletal material of Andrewsiphius and Kutchicetus, two Eocene cetaceans from India. Journal of Paleontology 83, 635-663.
Bats; hunters that see in sound
You are out in the dark, alone. You become aware of a distant rhythmical clicking noise. Sensing danger, you change direction and head for cover. Seconds later you are punched by wave after wave of sound that hits your body like a machine gun. The noise bears down on you, loud as an aircraft, faster and faster, blurring into a roar….
…You are a moth, taken by an echolocating bat.
Bats aren’t blind. Their eyes work, but they live in a different world, requiring an extra sense. Using high frequency sounds, also known as ‘biosonar’ or ‘echolocation’, they can see in the dark. We see, hear touch, smell and taste by picking up cues in our environment. Biosonar is completely different; there can be no signal without the call.

A bottlenose dolphin (Tursiops truncatus) with locator beacon; Persian gulf. This animal is part of a trained team of animals used by the US Navy for mine clearance in shipping lanes. Since most prey cannot detect high ... morefrequency sound, hunters using echolocation have a stealth surveillance system of near-military precision. Dolphin echolocation inspired naval underwater surveillance using sonar (Image: Wikimedia Commons)
This really is seeing in sound. Biosonar provides bats and some other animals, including dolphins, with a distinctive and sophisticated form of vision, giving detailed three-dimensional information over long distances in darkness or murky waters.
Bats illuminate their world with beams of directional sound
Most bats hunt using a range of frequencies, moving their heads to pulse sounds in different directions as they fly. Lower frequencies produce wider sound cones like a floodlight, and higher frequencies focus the beam like a spotlight.

Bats and other echolocators emit pulses of directional sound that echo back from a target, returning them a cone of sonic information. They control the length and width of this cone by altering the frequency of their ca... morells and how far they open their mouths when producing the pulse. Larger mouths and higher frequencies produce longer, narrower ‘visual’ sound cones (Image: ©Simon Crowhurst)
Although one lives in the forest and the other in the ocean, the way bats and dolphins use biosonar when hunting is almost identical. Dolphins, like bats, both shift the frequency of their clicks as they scan and lock onto prey, giving a characteristic ‘buzz’ of rapid calls as they take the target. Sound travels more quickly under water, so dolphin sonar operates over a longer range.
Bats project sound and receive echoes the way that eyes scan an image
Watch someone’s eyes as they examine something; their gaze jerks from one area to another. These are known as ‘saccade and fixate’ movements. They focus different parts of the image onto an area at the back of the eye called the fovea, which sees detail. ‘Saccade and fixate’ eye patterns are found in all animals with good vision, and is a defining characteristic for how eyes focus on details.

The human eye shifts its main focus in a series of ‘saccade and fixate’ movements. As this eye focusses on Sophocles’ hand, reflected light from the object lands on the fovea; the detail-harvesting area at the bac... morek of the eye. When looking at an object, our eyes move suddenly from one detail to another (the saccade), and then ‘fixate’ for a few moments onto these points of interest.Sophocles uses the idea of blindness in his story, ‘Oedipus Rex’, to illustrate how seeing is a highly active process, whether this is physical or metaphorical (Image: Composite of images from Wikimedia Commons)
When our eyes focus, they project an image onto an area at the back of our eyes known as the ‘fovea’. This zone is specialised to pick up high levels of detail. Any sense can be developed to pick up a high level of detail; we use sight, bats use sound, and star-nosed moles use touch.
Most bats hunt insects in the open air, listening for the returning echoes before making their next signal. They use a combination of head movements and focussed sound cones to gain information in a classic ‘saccade and fixate’ pattern. Wider head scanning and lower frequency calls provide a ‘wide angle’ view with less detail. Higher frequency calls and a narrow range of head movements create an ‘acoustic fovea’; this focuses their sonic gaze onto a small area and recovers detail, much like ‘zooming in’ using a macro lens.
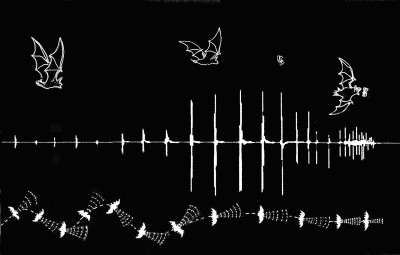
This artist’s impression shows the flight path (below) and behaviour (above) during the hunting sequence of a Common Pipistrelle bat (Pipistrellus pipistrellus), represented by the sound trace (centre). The process ha... mores distinct phases. During the initial ‘search’ (left), the call frequency is quieter (lower amplitude) and less frequent; this produces a wide angle sound cone. Upon detecting and approaching a target (centre), the hunter’s calls increase in volume and frequency, which focusses the acoustic gaze. As the hunter is locked onto its target (right), it produces a high frequency ‘buzz’. This highly focussed cone of sound allows the bat to perceive details, and manoeuvre very precisely as it closes in to take the prey.The time from detecting to taking the prey is less than a second. (Image: ©Simon Crowhurst)
Horseshoe bats use echolocation in a different way; an adaptation to hunting in and around the spatially variable (and hence acoustically complicated) ‘surface’ of tree and shrub canopies. They use mostly constant-frequency pulses, and listen for Doppler-shifted returning echoes. The cochlear membranes of their inner ears respond over a broad frequency range, but have particular sensitivity within a very narrow bandwidth (their acoustic fovea). When locking the sound pulse onto their prey, they alter their emitted call in order to keep the frequency of the returning echoes constant. This allows them to focus on the frequency modulations caused by the fluttering movements of large insects.
Echolocation reveals shape and form using ‘stereo’ images
Just as the brain uses the different view from our two eyes to build a stereo image, our brains use the differences between what is heard by our ears to understand something of the direction and distance of a sound source. Bats use this difference to understand and construct a ‘stereo image’ of their surroundings in three dimensions. This enables them to understand something of the shapes and textures of objects in their environment.
Some tropical bats are nectar feeders. Flowers hidden amongst the leaves are in an acoustically cluttered environment. Many flowering plants using bats to assist with their pollination have evolved floral structures that act as ‘sound beacons’. These are usually dish-shaped (parabolic) and bounce back an unique echo pattern that makes them become acoustically visible.

The Allen Telescope Array (ATA) built by the University of California, Berkeley and SETI (Search for Extra-terrestrial Intelligence). This offset Gregorian design reflects incoming radio waves caught by the large parabo... morelic dish onto the secondary parabolic reflector, which harvests the signal. The telescope is tuned to a frequency range from 0.5 to 11.2 GHz and will eventually have 350 antennae (Image: Wikimedia Commons)
Parabolic shapes also make excellent receivers. The Search for Extra-Terrestrial Intelligence (SETI) project’s radio telescope is made of many parabolic ‘ears’, listening for radio transmissions from outer space. Whilst we have invented technologies enabling us to hear bat calls and signals from beyond our planet, our own ears’ convoluted parabolic shape also captures sounds, and funnels them to our receiver; the ‘ear drum’ .
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Fenton, M.B. (2013) Questions, ideas and tools; lessons from bat echolocation. Animal Behaviour 85, 869-879.
Connor, W.E. and Corcoran, A. J. (2012) Sound strategies; the 65 million year old battle between bats and insects. Annual Review of Entomology 57, 21-39.
Ghose, K. et al. (2006) Echolocating bats use a nearly time-optimal strategy to intercept prey. PLoS Biology 4, 865-873.
Jakobsen, L. et al. (2013) Convergent acoustic field of view in echolocating bats. Nature 493, 93-96.
Land, M.F. (2011) Oculomotor behaviour in vertebrates and invertebrates In The Oxford Handbook of Eye Movements(S.P.Liversedge, I.D. Gilchrist and S. Everling, eds), pp. 3-15. Oxford University Press.
Ratcliffe J A et al. (2012) ‘How the bat got its buzz’ Biology Letters 9;20121031
Siebert, A M et al (2013) ‘Scanning behaviour in echolocating common pipistrelle bats (Pilistrellus pipistrellus) Plos One 8(4);e60752
Simon et al (2011) ‘Floral acoustics; conspicuous echoes of a disc-caped leaf attract bat pollinators’ Science 333: pp631-633
Schnitzler, H-U. and Denzinger, A. (2011) Auditory fovea and doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. Journal of Comparative Physiology, A 197, 541-559.
Surlykke, A. et al. (2009) Echolocating bats emit a highly directional sonar sound beam in the field. Proceedings of the Royal Society of London, B 276, 853-860.
von Helversen, D. and von Helversen, O. (2003) Object recognition by echolocation; a nectar feeding bat exploiting the flowers of a rainforest vine. Journal of Comparative Physiology, A 189, 327-336.
von Helversen, D. and von Helversen, O. (1999) Acoustic guide in bat pollinated flower. Nature 398, 759-760.
Elephants’ aquatic ancestors; just below the surface
Pausing on the bank, the lead female gathers her herd, ensuring that she is followed before she plunges in.
The smaller elephants swim, heads fully submerged. Others walk at first. All are snorkel-breathing through their trunks.
A lone male follows at a distance. He watches the tribe for a moment before he too enters the water.
Elephants are the largest land mammals, and are at home in hot, dry savannah environments. They migrate hundreds of miles in search of food, and when required will cross rivers, lakes, and even undertake marine excursions. They are surprisingly strong swimmers, and are the only mammals able to snorkel.
This provides a vital evolutionary clue to their past. Although we associate elephants with dry land, their bodies reveal clear evidence of an aquatic ancestry.
Fossils and DNA put elephants amongst the swimmers
DNA evidence:
Comparing DNA sequencesenables usto build molecular phylogenies, or ‘family trees’, defining the genetic relatedness between species. These phylogenies show that elephants’ closest living relatives are the fully aquatic sea cows; the dugongs and manatees.
Fossil evidence:

An artist’s impression of the semi-aquatic Moeritherium by scientific illustrator Heinrich Harder (1858-1935) (Image: Wikimedia Commons)
Water molecules (H2O) contain three isotopes of oxygen; 16O, 17O and 18O. The lighter forms evaporate more easily, slightly enriching lakes and oceans with ‘heavy’ water. This then can become incorporated into aquatic plants and the bodies of animals that graze on them. Analysing the ratio of ‘light’ 16O to ‘heavy’ 18O isotopes (i.e. d18O) in teeth from fossils of the extinct elephant ancestor Moeritherium, show that they contain a higher proportion of 18O than teeth from land grazing animals. This tells us that it ate mostly freshwater plants, suggesting its lifestyle was at least semi-aquatic.
Elephant embryos reveal adaptations to breathing under water

This photographic series of elephant foetuses shows the early appearance of the trunk. Natural History Museum, Maputo, Mozambique.An elephant foetus has detectable heart beats by day 80 of gestation, and the trunk start... mores to become visible at days 85-90. Overall, gestation is approximately 660 days (Image Wikimedia Commons)
Elephants can snorkel because of their elongated trunks, and elastic connective tissues that fill the space between their lungs and their body wall. Both of these features appear together early in the foetus, suggesting that they were important for survival of the elephant’s ancestors.
As in other large mammals, elephants’ blood pressure is high; this is needed to keep the brain well supplied with oxygenated blood. Under water, the differences between the pressure of inhaled air (atmospheric; 0mmHg) and the blood pressure in the capillaries (150mmHg) would be enough to rupture blood vessels and the delicate linings of the lung. Their elastic connective tissues act as shock absorbers, protecting the lungs against such damage.
Elephants’ testes are inside the body like other aquatic mammals

A male western grey kangaroo (Macropus fuliginosus) foraging near Port Douglas, Queensland. In hot weather, male kangaroos lower their scrotal sac away from the body; this helps keep these organs at a cooler temperature... more and avoids damaging their sperm (Image: Wikimedia Commons)
The location of a mammal’s testes is related to temperature. Excessive heat or cold will kill mammal sperm (including humans), causing sterility. Hence in puberty, boys’ testes descend into sacs that are slightly cooler than the interior of their bodies. However aquatic mammals continue to carry their testes inside their abdomen to protect them from cold. We would expect land-dwelling elephants’ testes to be carried in a scrotum. Instead, their testes remain inside the body like those of aquatic mammals.
Early in the foetal development of both male elephants and dugongs, an artery develops which directly connects their kidneys and testes. In contrast, the testes of most land mammals have a less direct connection, allowing them to descend into a scrotum during puberty. This suggests that like modern dugongs, the elephants’ ancestors lack a scrotum.
Aquatic mammals including dugongs have a plexus of blood vessels that cool their internal testes and uterus through a counter-current heat exchange mechanism. Testicular cooling by a blood plexus has not yet been investigated in elephants; however in the absence of heat stress, they maintain a body temperature of around 34-36⁰C, which is similar to the temperature of the scrotum in most other land mammals.
Elephants ears close for swimming

This skull (formerly from a zoo elephant, kept at Basle in Switzerland) has had a front (sagittal) section removed, to show the honeycomb of air cavities inside the bones. Elephants hear low frequencies (infrasounds) tr... moreansmitted through the ground and conducted to their skull and inner ears through the bones of their front legs. These sounds are amplified by resonating in these inner bone chambers (Image: Wikimedia Commons)
After swimming we often have to shake our head to clear water from our ears. However elephant ears have a unique sphincter-like muscle that closes the ear canal and prevents water entry when swimming. This also creates a sealed ‘acoustic tube’, which works with the aerated bones and ‘acoustic fat’ lenses of the elephant skull to amplify low frequency ‘infrasound’. Dugongs have similar aerated skull bones and fatty deposits; again implying that they share an aquatic ancestor.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Fowler, M.E. and Mikota, S.K. (eds) (2006) Biology, Medicine, and Surgery of Elephants. Blackwell.
Gaeth, A.P. et al. (1999) The developing renal, reproductive and respiratory systems of the African elephant suggest an aquatic ancestry. Proceedings of the National Academy of Sciences, USA 96, 5555-5558.
Hildebrandt, T. et al. (2007) Foetal age determination and development in elephants. Proceedings of the Royal Society of London, B 274, 323-331.
Johnson, D.E. (1978) The origin of island mammoths and the Quaternary land bridge history of the Northern Channel Islands, California. Quaternary Research 10, 204-225.
Johnson, D.E. (1980) Problems in the land vertebrate zoogeography of certain islands and the swimming powers of elephants. Journal of Biogeography 7, 383-398.
Liu, A.G.S.C. et al. (2008) Stable isotope evidence for an amphibious phase in early proboscidean evolution. Proceedings of the National Academy of Sciences,USA 105, 5786-5791.
McKenchie, A.E. and Mzilikazi, N. (2011) Heterothermy in Afrotropical mammals and birds: a review. Integrative and Comparative Biology 51, 349-363.
O’Connell-Rodwell, C.E. (2007) Keeping an ear to the ground: seisemic communication in elephants. Physiology 22, 287-294.
Poulakakis, N. et al. (2006) Ancient DNA forces reconsideration of evolutionary history of Mediterranean pygmy elephantids. Biology Letters 2, 451-454.
Poulakakis, N. and Stamatakis, A. (2010) Recapitulating the evolution of Afrotheria: 57 genes and rare genomic changes consolidate their history. Systematics and Biodiversity 8, 395-408.
Seiffert, E.R. (2007) A new estimate of Afrotherian phylogeny based on simultaneous analysis of genomic, morphological and fossil evidence. BMC Evolutionary Biology 7, 224.
Springer, M.S. et al. (2004) Molecules consolidate the placental mammal tree. Trends in Ecology and Evolution 19, 430-438.
Weissenbrock, N.M. et al. (2012) Taking the heat: thermoregulation in Asian elephants under different climatic conditions. Journal of Comparative Physiology, B 182, 311-319.
West, J.B. Why doesn’t the elephant have a pleural space? Physiology 17, 47-50.
West, J.B. et al. (2003) Fetal lung development in the elephant reflects the adaptations required for snorkelling in adult life. Respiratory Physiology and Neurobiology 138, 325-333.
West, J.B. (2001) Snorkel breathing in the elephant explains the unique anatomy of its pleura. Respiration Physiology 126, 1-8.


