Entering a body is much like entering a country.
At the border, agents verify the identity of those seeking to pass, and establish their status as ‘native’, a ‘naturalised resident’, or ‘alien’. Clarity about the identity of these travellers allows them to be appraised as ‘friend’ or ‘foe’.
The body of a nation operates best with safety measures in place. Its borders are a first line of surveillance that ascertains when to mobilise defences.
Border checks in some countries are more efficient; they deploy new technologies to implement higher orders of monitoring and identification.

Scanning electron microscope image of a neutrophil, a cell from the innate immune system (yellow), engulfs anthrax bacteria (orange); scale bar = 5 micrometres.Body cells that experience stress, wounding, or sense the p... moreresence of bacteria, produce cytokines and other signals that trigger the innate immune response, and attract immune cells into the tissues.Some of these cells (‘phagocytes’) like this neutrophil, engulf and digest the invaders, whilst others (‘granulocytes’) secrete granules of cytokine, histamine and various types of oxygen free radicals, all of which enhance the inflammation response. Proteins in the blood, the ‘compliment system’, also condense onto and coat unwanted bacterial invaders and other particles. This targets them for destruction by phagocytes, or removal from the blood stream in the spleen (Image: Wikimedia Commons)
Typically we think of the immune system as a defence mechanism; the forces we mobilise to fight disease. Animals, plants, fungi and microbes all have a form of this type of defence. Even bacteria have a version of immunity, using enzymes that can react to and then digest viral proteins.
Immune responses in animal systems are thought of as having two levels of action.
Innate immunity
First, ‘innate immunity’ provides a spectrum of responses which use secreted proteins and dedicated cell types to target common components of a broad range of diseases.
Amongst animals, invertebrate bodies are relatively simple. In contrast, vertebrates have increased genetic and physical complexity, more sophisticated sensory perceptions and cognitive processing and, in some clades, enhanced social organisation and communication. This increased complexity is reflected in the vertebrate immune system.
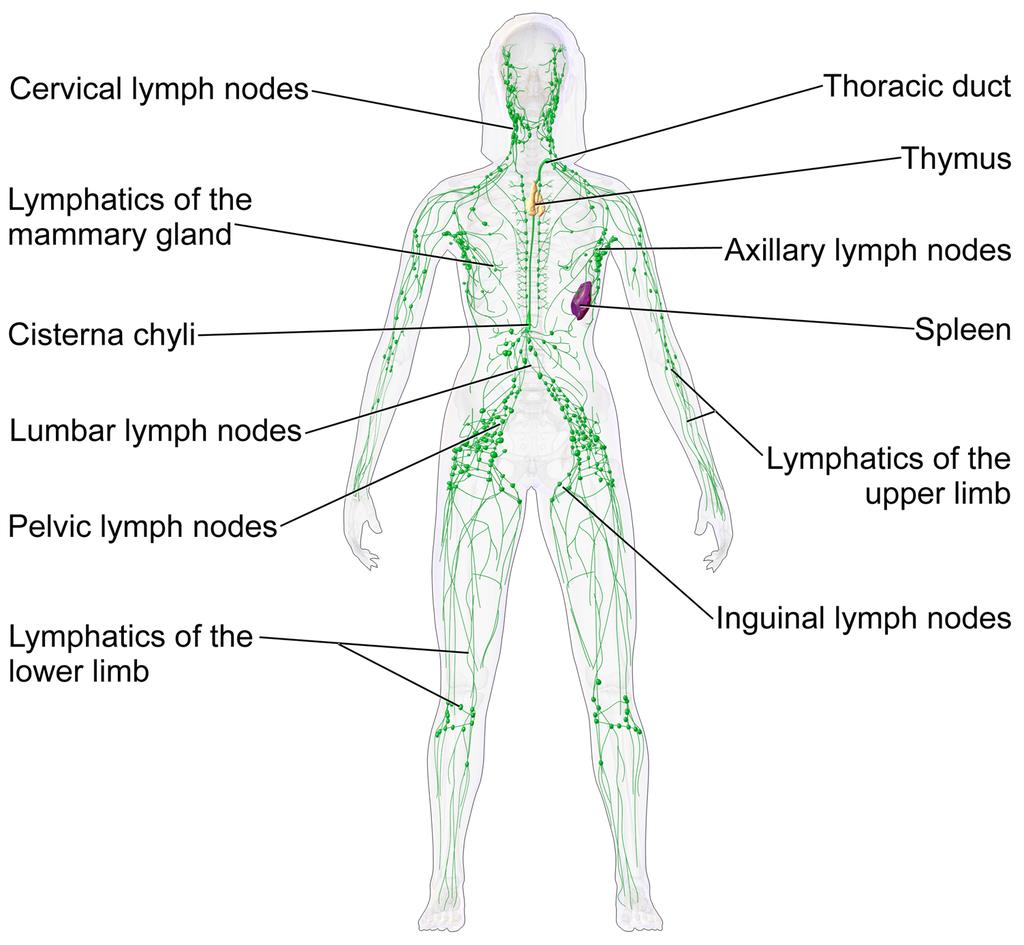
The lymphatic system of a human female. This network of vessels allows immune system cells rapid access to all areas of the body. Lymph vessels interconnect with the blood circulation at the heart and lymph nodes.Two or... moregans are particularly important in this network.• The thymus gland polices antibody-producing adaptive immune system cells, filtering out those which are too close to recognising native body proteins.• The spleen synthesises antibodies in its white pulp, and releases these proteins into the blood stream, where they bind to foreign proteins, such as those present on the surface of bacteria. This targets cells and other debris in the blood. The spleen removes antibody-coated bacteria and old red blood cells from the circulation. It also acts as a reservoir for monocytes, immune cells which later develop into phagocytic (non-specific engulfing) cells (Image: Wikimedia Commons)
Adaptive Immunity
The second form of immune response, known as ‘adaptive immunity’, refers to the ability to identify and target specific invaders. In vertebrates, this immune function first appeared in jawed fish.
In broad terms, it involves three phenomena.
– A new immunological organ (the thymus).
– A repertoire of specialised lymphocyte cells including T-cells (matured in and released from the thymus gland) and B-cells (released from the bone marrow), some of which produce antibodies.
– The production of a ‘self-identity’ signature, the ‘Major Histocompatibility Complex’ (MHC) proteins, in all body cells.
The community of bacteria associated with our gut, lungs and other surfaces comprise the first line of defence. This community of microbes (our ‘microbiome’) out-competes many harmful agents (pathogens).

Like the gut, our lung linings have a microbiome. Both ’friendly’ microbes and infective agents (here Streptococcus pneumoniae and Pseudomonas aeruginosa) produce proteins and other chemicals that act as signals. C... moreells lining our lungs act as signal receivers and recognise these chemicals, producing cytokines and other cues which relay messages to the immune system.(Image: Wikimedia Commons)
If our body cells become infected or damaged, they release stress signals such as oxygen and nitrogen free radicals and various peptides (short strings of amino acids) such as cytokines and defensins. These cues act as messages to the immune system, potentially triggering inflammation and other chemical defences which attract immune cells into the tissue by chemotaxis.
The microbial community also produces these immune-stimulating signals, along with brain-active chemicals (neurotransmitters). In addition, these signals are also generated, recognised by, and responded to by immune system cells.

Diagram of an antibody. These immune system proteins are a complex of four separate protein chains. In this diagram, blue regions are consistent whilst the yellow regions are variable. They are secreted into the blood s... moretream or gut. Adaptive immune cells use them as ‘antennae’. When this signal receiver encounters an antigen that is a good fit, this triggers an adaptive response. All proteins interact by shape. Antibodies have a recognition region which is highly variable. The strongest immune reactions provide a ‘best fit’ to this three-dimensional molecular jigsaw. (Image: Wikimedia Commons)
Our neurons and immune cells both respond to hormones within our bodies, since chemically these are close to neurotransmitters. This means that our brain, hormonal system, sensory nerves and extended microbial community all share a common chemical language with the innate immune system, present in all animals.
The greater the biochemical diversity of cell populations in a body, the more opportunities there are for bacteria or viruses to find novel ways of attacking these cells. For this reason, adaptive immune system cells themselves are vulnerable to certain types of disease. For instance AIDS (Acquired Immuno-Deficiency Syndrome), caused by the Human Immuno-deficiency Virus (HIV), specifically targets and infects the adaptive immune system’s T-cells, using the very mechanism that allows these cells to detect infection – their surface antibodies.
Potentially then, adaptive immunity provides a much more sophisticated and versatile immune mechanism. For vertebrates with their adaptive system to be ultimately more vulnerable to certain infections than the innate-only immunity of invertebrates seems bizarre and inefficient. Yet adaptive immune systems have evolved independently twice in vertebrates. This suggests that the adaptive mechanism must convey some survival advantages, but disease resistance may not be the primary role which has driven its evolution.
How does the system work, and what is the real purpose of adaptive immunity?
Adaptive mechanisms give our immune system a ‘memory’
In vertebrates, ‘adaptive immunity’ adds an additional higher-order function to the innate immune system by providing a means to both recognise and ‘remember’ specific diseases. To do this, they use a protein recognition system; these are the familiar antibodies.
These proteins have a shape-specific region which fits the shape of part of a foreign protein (an antigen) like a key in a lock. An antigen is a molecule that is capable of triggering an immune response; this can be from a foreign source (e.g. a virus) or produced by an unhealthy body cell, e.g. a cancer cell.

The adaptive immune system “adapts” to infections that get past our innate defences. Phagocytic macrophage (innate system) cells behave like amoebae, and can engulf and digest foreign bacteria. These ce... morells become ‘antigen presenting cells’ (APC), and displaying short pieces of bacterial protein (the antigen) on their cell surfaces, along with a self-signal, the ‘Major Histocompatibility Complex’ class II protein (MHC2). The MHC tells the immune system that this cell is a messenger, not an invader. These two proteins together activate the adaptive immune system.There are two types of adaptive response. First, T lymphocytes (T cells) display an antibody on their surface that recognises the foreign fragment on an APC. They then produce other surface proteins (here CD4+) that signal a change of identity, turning them into ‘‘helper’ cells. Helpers can provoke macrophages or B lymphocytes (B cells) to act. An activated B cell releases antibodies into the blood stream that ‘seek out’ and identify the invader. T cells can also become ‘killers’ that recognise and destroy infected body cells. (Image: Wikimedia Commons)
Adaptive immune cells called T-cells produce antibodies and ‘display’ them from their cell surfaces, using them as ‘antennae’ to detect invaders. Phagocytic (engulfing) innate immune cells can act as antigen presenting cells, displaying foreign protein fragments (antigens) on their surfaces. T-cells whose antibody ‘fits to’ this antigen can then be triggered to respond.
Triggered T-cells divide multiple times, so producing identical clones of themselves. This increases the magnitude of the body’s immune response to the infection. Activated T-cells relay the signal in turn to adaptive B-cells, triggering them to secrete large amounts of antibody proteins specific to this foreign antigen into the blood. These bind to the foreign antigens, coating the virus particles or foreign bacteria in antibodies. This targets the innate system’s macrophages to ‘engulf and destroy’.
Immune system cells interact with each other in a similar way to nerve cells
The association between the antigen presenting cell and the activating T-cell is very close, and the trigger mechanism transmits a one-way signal between the cells. This is very like how a nerve synapse transmits a signal to the next nerve. It is therefore entirely reasonable to call it an ‘immunological synapse’.
This between-cell interaction, which here involves a specific recognition between the immune cells, arises from an ancient, general mechanism of between-cell communication that allows signal-transmitting contacts to form. Activated T-cells initially show pulses of electrical activity, produced by a release of calcium ions (which carry an electrostatic charge) into the cell matrix (cytoplasm).
Synapses are stable contacts that form between two distinct cells that allow for information transfer through directed secretion. Synapses between neurons in the brain form relatively stable contacts, although these remain ‘plastic’, able to be recruited into new neural networks and adopted for new purposes.
In the immune system, ‘synapse’ formation between for example an antigen presenting cell and a T-cell, provokes the T-cell to initiate a signal relay transmitted to other cells which produces a range of specific ‘actions’. These responses, resulting in defences that target the specific foreign antigen, are produced by cells further downstream of this initial ‘synapse’.

When a patient is first vaccinated, the adaptive immune system raises a response against the foreign antigens. These cells persist at a high level for several weeks, then the antibody level and effector T-cell activity ... moregradually declines. When a patient is re-vaccinated, a stronger response results. Selection favours T-cells with small differences in antibody binding that give a stronger reaction. These highly specific clones remain in the serum and lymphatic tissues and provide protective immunity against reinfection by the same agent. This gives a longer term immunological memory; later reinfection leads to a rapid increase in antibody production and effector T cell activity, which often prevents the disease from being apparent (Image: Wikimedia Commons)
Vaccinations activate our body ‘memory’
By cloning and maintaining a population of cells with disease-specific antibodies, our immune system ‘remembers’ how it responded to the disease. A second exposure produces a refinement of the accuracy of the recognition mechanism, and raises a population of ‘memory T-cells’ that persist in the body for much longer. This is the basis of vaccination. Exposing our immune systems to proteins from the infective agent, via an injection, allows us to develop resistance in a controlled, safe way. Our bodies raise a population of immune cells that ‘remember’ how to recognise these foreign proteins. If we later meet the disease, these ‘memory cells’ activate, enabling us to more quickly overcome the infection and contract only a mild version of the disease, or perhaps experience no symptoms at all.
These mechanisms suggest that adaptive immunity should provide enhanced resistance to diseases beyond what the innate response alone can deliver. In practice, however, invertebrates seem to be vulnerable to fewer diseases than we are. This may be a result of their simpler and less diverse profile of cell and tissue types.

Diagram of an HIV particle. Using cell surface antibodies as an infective agent identification mechanism means that immune cells can in turn become ‘visible’ to these pathogens. The Human Immunodeficiency Virus (HIV... more) which causes Acquired Immuno-defficiency Syndrome (AIDS) specifically identifies T-cells. This virus is a master of disguise; it uses a similar gene shuffling mechanism as are used to produce variable proteins on the surface of its viral capsule. This changeability makes the virus seem to always be something new – this is the reason that the immune system has difficulty recognising and neutralising it, and also is why the virus has been so difficult to target using a vaccine. The interaction between an infection and the host immune system is usually like a predator prey relationship. The immune system is the ‘predator’, chasing down to consume the infection (the ‘prey). In the case of HIV, these roles are unclear. Is the virus pursing the immune cell, or is the immune cell pursuing the virus? (Image: Wikimedia Commons)
Why would vertebrates evolve this expanded complexity in their immune system?
Every living thing, including plants and bacteria, possess an immune system of some kind. What we recognise as innate immunity is found in plants and also throughout the animal kingdom. Adaptive immunity raises the degree of complexity. Significantly, it has evolved twice independently within the vertebrates; in jawed vertebrates (sharks to humans) and also in the more ‘primitive’ jawless clades. Certain invertebrate groups (e.g. snails and insects), and indeed also bacteria, have a quasi-adaptive system, using specific, cross-reactive molecules, although these systems are not as sophisticated as that found in the vertebrates.

A river lamprey (Lampetra fluviatilis) from the German North Sea. The immune systems of this and other jawless fish such as the hagfish have been found to contain adaptive cells which act in a similar way to T- and B- l... moreymphocytes of the jawed vertebrates. Here the membrane proteins acting as signal receivers are different, based on receptors containing leucine-rich motifs. As in their jawed cousins, the immune responses of lampreys produce a clonal increase of specific disease-detecting cells (Image: Wikimedia Commons)
Studies in the jawless fish, lamprey and hagfish, show that a very different form of adaptive system has arisen than is found in jawed vertebrates, co-opted from a different set of genes and proteins. In contrast, their immune molecules include proteins with repeated sections in the chain which have multiple residues of the amino acid leucine (termed ‘Leucine Rich Repeats’, or LRRs). A form of LRR-based adaptive immunity is also found in plants.
Jawed vertebrate adaptive immunity however is rather more developed than that of the jawless fish. Jens Rolff makes two intriguing suggestions as to what may have driven its evolution.
i. Vertebrate bodies are larger and more complex than invertebrates, providing a new set of potential habitats for parasites. Rolf suggests that an evolutionary ‘arms race’ may have occurred between vertebrates and a group of parasitic Platyhelminthes, which include tapeworms and liver flukes. These parasites began to diversify after the evolutionary divergence between jawed and jawless fish.
The surface of these parasites is highly resistant to our immune defences, suggesting that they co-evolved in tandem with an immune-competent host. The increasingly more aggressive and targeted immune response required from the host to expel these parasites may have selected for the direct recognition mechanism and targeted responses of adaptive immunity. This theory also implies that the adaptive components of the immune system originally evolved in association with the gut.
ii. Vertebrates have higher metabolic rates than invertebrates. This allows for greater rates of activity and access to a wider range of ecological habitats. Higher energy bodies require more sophisticated brains and nervous systems, and have higher maintenance demands.

Scheme showing a DNA ligase-I enzyme (in color) repairing a break in DNA. DNA damage, caused by environmental factors and normal metabolic processes, occurs at a rate of 1,000 to 1,000,000 molecular lesions per cell per... more day. Without maintenance to mend such breaks, cells can malfunction, die, or become cancerous. Ligases catalyse the crucial step of joining the breaks in the spiralling DNA strand. To do so they require energy, in the form of either Adenosine triphosphate (ATP) or Nicotinamide adenine dinucleotide (NAD+) as a cofactor (Image: Wikimedia Commons)
Rolff suggests that the adaptive immune system is part of the normal homeostasis of a higher energy metabolism. Such regimes also produce higher concentrations of oxygen free radicals and other stress-associated compounds which can damage DNA and cause cancers. High energy vertebrate bodies therefore require a means of self-monitoring. The adaptive immune system may provide just such a set of ‘internal eyes and ears’.
How does the adaptive immune system function in vertebrates?
Our immune system discerns ‘friend’ from ‘foe’, ‘self’ from ‘non-self’ and monitors the status and identity of our own body cells. The adaptive system provides specific ways to identify and monitor foreign and native body cells.
An immunological synapse relay is triggered when for example a T-cell antibody recognises a foreign protein on an ‘antigen presenting cell’. This and other types of immunological synapse require a class of proteins related to antibodies; the ‘Major Histocompatibility Complex’ (MHC) proteins.
Vertebrates produce and display MHC proteins on their cell surfaces. During an infection, if a virus or microbial pathogen enters a body cell, it becomes invisible to the immune system. Infection, however, alters the health of the cell, reducing its surface MHC production. Reduced or absent MHC triggers ‘Natural Killer’ (NK) cells to form an immunological synapse with them, this time triggering the destruction of the infected body cell, and helping to reduce the spread of the disease.

As a cell is first infected by a microbial pathogen, (as in this diagram), it quickly mobilises its protein-dismantling enzymes (the ‘proteasome’) to digest the foreign proteins into short fragments (peptides). Spec... moreialised pores (‘Transport of Alien Protein’ or TAP channels) capture the fragments and shunt them into the endoplasmic reticulum, where the cell assembles its own proteins. Here the fragments are bound to ‘Major Histocompatibility Complex’ class 1 (MHC I) proteins. These are transported to the cell surface where they protrude like a beacon, presenting the foreign protein to the innate immune system’s ‘Natural Killer’ cells (Image: Wikimedia Commons)
MHC proteins also act as antigen presenting molecules. At an early stage of infection, the cell’s own defences may digest some of the invading pathogen’s proteins. These foreign antigens can be bound by MHC molecules, and presented on the cell surface. Again, this triggers NK cells to target this body cell for destruction.
Antigen-presenting phagocytic immune cells also do this using a different class of MHC protein, which avoids them activating the NK cells.
Recent research has also shown an unexpected role for MHC proteins in neuronal plasticity. Brain neurons require MHC class I molecules to establish new neural networks. Along with a range of other immune system receptors, these molecules are critical for the construction of new connections and the remodelling of synapses. This suggests that the transmission of a signal across the neural synapse and the immunological synapse are likely using the same mechanism.
What does this suggest as the primary role of adaptive immunity?

The ‘molecules of emotion’ with their effects. The mood effects that result from their deficiency in italics. These neurotransmitters are the same chemical language as is used by cells of the immune system (Image: W... moreikimedia Commons)
Candace Pert’s work demonstrated that immune cells both respond to and produce the same neurotransmitter signals as brain neurons. She initially found opiate receptors from the brain in immune system cells. Opiates are drugs that trigger the body’s responses to endorphins, the body’s natural ‘feel good’ chemicals. Pert showed that receptors for neurotransmitters which affect our mood; the ‘molecules of emotion’ including b -endorphins and serotonin, are present in immune system cells. Immune cells also manufacture these same chemicals.
Immune cells are everywhere in the body. Brain-based phenomena such as depression impact the function of the immune system such that our level of disease resistance rises and falls with our moods. In at least chemical terms, these cells are operating in conjunction with our neural network. Pert’s results reveal that through the immune system, our minds occupy the full sensory space of our bodies, and are not limited to our brains. By the same route, immune cells convey the chemical implications of our emotional state into every cell in our body. This means that the way we think has a direct impact on our health.
Adaptive immune cells recognise stress signals, neurotransmitters, and foreign proteins. Foreign antigen recognition activates the immunological synapse, and triggers the adaptive immune response. Recent research has shown that the MHC proteins, essential for this response, are also involved in the functioning of nerve synapses in the brain. This implies that the two systems are really both part of the same ‘neural super-organ’.
This ‘organ’ has flexibility as to where it focusses its attention. Release of cytokines and other stress signals draw immune cells into tissues where their surveillance is most required. This roving internal sense organ has been referred to by Enzo Ottavani and Claudio Franceschi as an ‘immune-mobile brain’ whose ‘eyes and ears’ are our adaptive immune cells.

Poster, emphasising the importance of surveillance, issued by the office for Emergency Management in the United Kingdom during the Second World War (Image: Wikimedia Commons)
This surveillance system cross-reacts with and can mutually influence the body’s hormonal system and gut microbiome. Edwin Blalock has suggests a primary role for the immune system as a sense organ; truly our ‘sixth sense’, which is able to detect stimuli not recognised by the central or peripheral nervous system. This information, the chemical language of the body’s bacterial community, is translated into a cognitively accessible form by the antennae of the adaptive immune system.
This provides a picture of the brain and immune system as forming an integrated ‘body-mind’. This system’s sensory perceptions, thoughts, emotions and behavioural responses impact the physical body at the biochemical level. This ‘embodiment’ idea has also become apparent through recent insights into the actions of mirror neurons and the neural basis of human language.
Having a conversation in the same language synchronises our minds. The brain and immune system translate this into hormonal and other chemical cues of emotion.
This suggests that our immune system is one part – a subconscious part – of our overall consciousness. This consciousness is integrated into our ‘body-mind’. Through sharing our thoughts with others, our awareness operates beyond the body at a higher level, and as we do this, we connect at the level of our chemistry.
Conclusions
- Innate immunity is general, and present in all cells in some form, from bacteria to humans.
- Adaptive immunity, where the organism raises a specifically cross-reacting chemical response to an infection, has evolved multiple times in multicellular life forms, including twice in vertebrates. Characteristics of adaptive immune systems in vertebrates are the appearance of cross-reacting proteins (antibodies) produced by specialised immune cells, a self-identifier (the major histocompatibility complex) in all body cells, and a thymus gland, which is a new organ within the lymphatic system.
- The driver of immune system evolution in vertebrates is not primarily defence. Factors that have driven the evolution of immune complexity in the vertebrate system (relative to invertebrates) may include (i) evolutionary ‘arms races’ with gut parasites, and (ii) an increased need to monitor the metabolic by-products of the higher energy vertebrate body.
- Immune complexity seems to be linked to the complexity of the overall organism, and fulfils a self-surveillance role. The increased complexity of vertebrate systems has in fact made them vulnerable to a wider range of infections than invertebrates.
- Vertebrate immune cells, neurons, and body cells all share a common chemical language; a language also used by the associated microbes that live on and around the vertebrate body (the microbiota).
- Immune cells form synapses, using a similar between-cell communication apparatus that neurons use to form their synapses. However the immune synapses are more transient, and this component of the body’s relay network is able to migrate to tissues where it is needed, augmenting the body’s surveillance in this area.
- Brain-body communication is two-way. The immune system integrates our thoughts and emotional state with our body, as well as informing the mind of the status of the body’s cells and tissues.
Text copyright © 2015 Mags Leighton. All rights reserved.
References
Abedon, S.T. (2012) Bacterial ‘immunity’ against bacteriophages. Bacteriophage 2, 50–54.
Bellinger, D.L. and Lorton, D. (2014) Autonomic regulation of cellular immune function. Autonomic Neuroscience 182, 15-41.
Bhalla, A.K. (1989) Hormones and the immune response. Annals of the Rheumatic Diseases 48, 1-6.
Bhat, R. and Steinman, L. (2009) Innate and adaptive autoimmunity directed to the central nervous system. Neuron 64, 123-132.
Blalock, J.E. (2005) The immune system as the Sixth Sense. Journal of Internal Medicine 257, 126-138.
Blalock, J.E. (2002) Harnessing a neural-immune circuit to control inflammation and shock. The Journal of Experimental Medicine 195, F25-F28.
Boulanger, L.M. (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64, 93-109.
Brenneman, D.E. et al. (1988) Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature 335, 639-642.
Bromley, S.K. et al. (2001) The immunological synapse. Annual Review of Immunology 19, 375-396.
Carr, D.J. et al. (1989) Hormones common to the neuroendocrine and immune systems. Drug Design and Delivery 4, 187-195.
Chacon, M.A. and Boulanger, L.M. (2013) MHC class I protein is expressed by neurons and neural progenitors in mid-gestation mouse brain. Molecular and Cellular Neuroscience 52, 117-127.
Chapman, R.C. et al. (2008) Pain and stress in a systems perspective; reciprocal neural, endocrine and immune interactions. The Journal of Pain 9, 122-145.
Cooper, E.L. (2010) Evolution of immune systems from self-not self to danger to artificial immune systems (AIS). Physics of Life Reviews 7, 55-78.
Elenkov, I.J. et al. (2000) The sympathetic nerve – an integrative interface between two super-systems; the brain and the immune system. Pharmacological Reviews 52, 595-638.
Goebel, M.U. et al. (2002) Behavioral conditioning of immunosuppression is possible in humans. FASEB Journal 16, 1869-1873.
Herbein, G. and Varin, A. (2010) The macrophage in HIV-1 infection; from activation to deactivation? Retrovirology 7, 33.
Malagoli, D. and Ottaviani, E. (2010) Life is a huge compromise; is the complexity of the vertebrate immune-neuroendocrine system an advantage or the price to pay? Comparative Biochemistry and Physiology, A 155, 134-138.
Ottavani, E. et al. (1988) The neuro-immunological interface in an evolutionary perspective; the dynamic relationship between effector and recognition systems. Frontiers in Bioscience 3, 431-435.
Ottovani, E. et al. (2007) Common evolutionary origin of the immune and neuroendocrine systems; from morphological and functional evidence to in silico approaches. Trends in Immunology 28, 497-502.
Ottaviani, E. and Franceschi, C. (1996) The neuroimmunology of stress from invertebrates to man. Progress in Neurobiology48, 421-40. Erratum in: Progress in Neurobiology 49, 285.
Pert, C. (1997) Molecules of Emotion; Why You Feel the Way You Feel. Schribner.
Pert, C. and Marriot, N. (2006) Everything You Need to Know to Feel Go(o)d. Hay House.
Polianova, M.T. et al. (2005) Chemokine receptor-5 (CCR5) is a receptor for the HIV entry inhibitor peptide T (DAPTA). Antiviral Research67, 83-92.
Pollitica, M. et al. (2007) Profound anti-HIV-1 activity of DAPTA in monocytes/macrophages and inhibition of CRR5-mediated apoptosis in neuronal cells. Antiviral Chemistry and Chemotherapy 18, 285-295.
Quan, N. and Banks, W.A. (2007) Brain-immune communication pathways. Brain, Behaviour and Immunity 21, 727-735.
Raberg, L. et al. (2002) Basal metabolic rate and the evolution of the adaptive immune system. Proceedings of The Royal Society of London, B 269, 817-821.
Rolff, J. (2007) Why did the acquired immune system of vertebrates evolve? Developmental and Comparative Immunology 31,476-482.
Rosi, S. et al. (2005) Chemokine receptor 5 antagonist D-ala-peptide T-amide reduces microglia and astrocyte activation within the hippocampus in a neuroinflammatory rat model of Alzheimer’s disease. Neuroscience 134, 671-676.
Ruff, M.R. et al. (2003) Update on D-ala-peptide T-amide (DAPTA); a viral entry inhibitor that blocks CRR5 chemokine receptors. Current HIV Research 1, 51-67.
Salles, A. et al. (2013) Barcoding T cell calcium response diversity with methods for automated and accurate analysis of cell signals (MAAACS). PLoS Computational Biology 9, e1003245.
Shatz, C.A. (2009) MHC class I: an unexpected role in neuronal plasticity. Neuron 64, 40-45.
Smith, E.M. and Blalock, J.E. (1988) A molecular basis for interactions between the immune and neuroendocrine systems. International Journal of Neuroscience38, 455-64.
Wahl, S.M. et al. (2006) HIV accomplices and adversaries in macrophage infection. Journal of Leukocyte Biology 80, 973-983.
Weigent, D.A. et al. (1990) Bidirectional communication between the neuroendocrine and immune systems. Common hormones and hormone receptors. Annals of the New York Academy of Sciences579, 17-27.
Wrona, D. (2005) Neural–immune interactions; an integrative view of the bidirectional relationship between the brain and the immune systems. Journal of Neuroimmunology 172, 38-58.
Yong, V.W. and Rivest, S. (2009) Taking advantage of the systemic immune system to cure brain diseases. Neuron 64, 55-60.
Yuan, S. et al. (2014) Amphioxus as a model for investigating evolution of the vertebrate immune system. Developmental & Comparative Immunology (in press).